-

Predicting Reaction Products: Simplified Answers and Insights
This article offers answers and explanations for a worksheet focused on predicting chemical reactions' products, enhancing understanding of stoichiometry, synthesis, decomposition, and combustion reactions.
Read More » -

5 Essential Answers for Solution Stoichiometry Worksheet
This worksheet provides answers and detailed explanations for solution stoichiometry problems, aiding students in understanding chemical reactions in solutions.
Read More » -
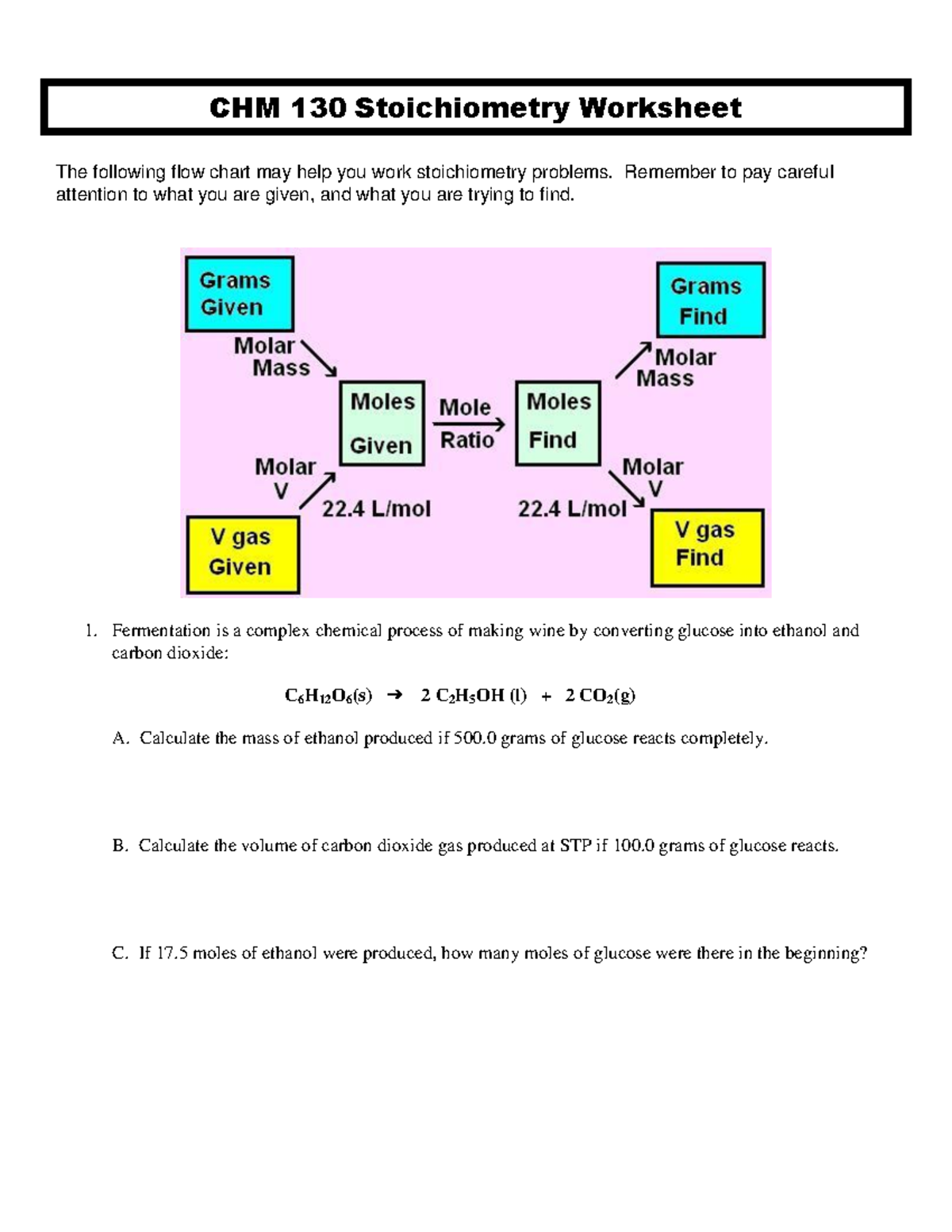
Master Stoichiometry: Chm 130 Worksheet Guide
This worksheet aids students in understanding and practicing stoichiometry calculations in CHM 130, covering key principles like mole ratios, limiting reactants, and yield calculations.
Read More » -

5 Key Answers to Mole Concept Worksheet Revealed
This article provides answers and explanations to help students understand the concept of moles in chemistry through a worksheet.
Read More » -

Master the Mole: Volume Calculations Simplified
Chemistry worksheet focusing on understanding the relationship between moles, volume, and gas laws.
Read More » -
Mastering Stoichiometry: Your Ultimate Guide
A beginner's guide to understanding the quantitative relationships in chemical reactions, focusing on balancing equations, mole ratios, and mass-mole conversions.
Read More » -

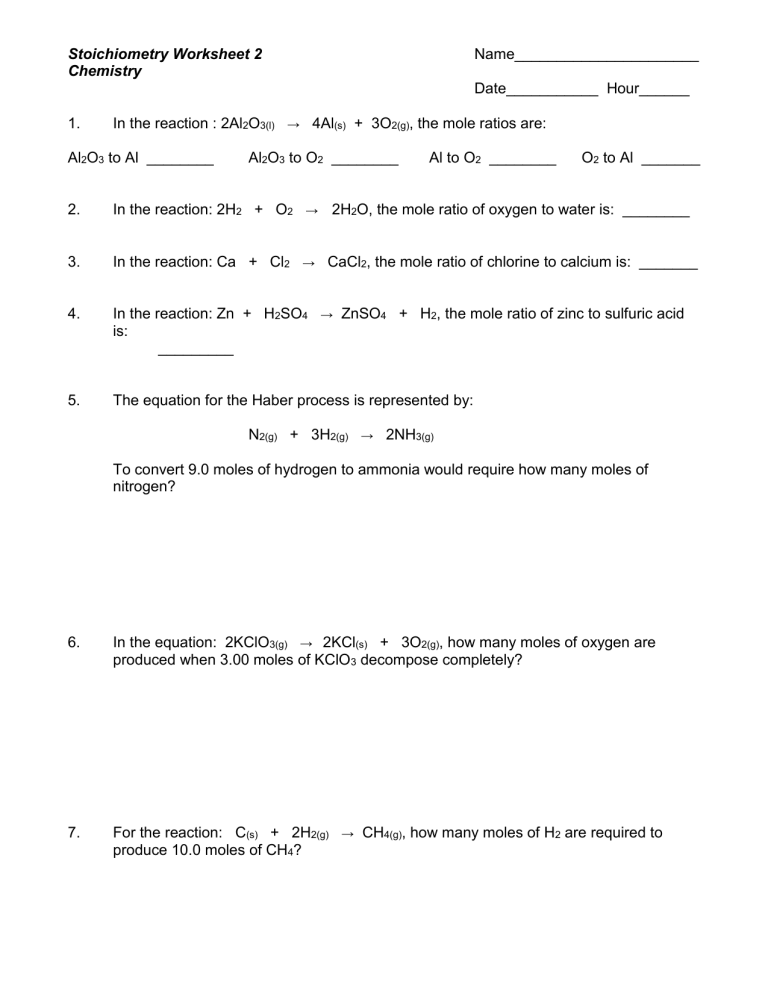
Stoichiometry Worksheet 2: Top Answers Revealed
Answer key for Stoichiometry Worksheet 2, providing detailed solutions to chemical equation problems.
Read More » -

Stoichiometry Made Simple: 5 Quick Worksheet Solutions
This worksheet provides answers to stoichiometry problems, helping students understand chemical reaction calculations.
Read More » -

5 Stoichiometry Problems Solved Easily
This worksheet provides practice problems for students to improve their understanding and skills in stoichiometry, involving calculations with chemical equations and mole ratios.
Read More » -

Solution Stoichiometry Worksheet: Master with Answers
A worksheet providing practice problems and solutions on calculating the amounts of substances in chemical reactions using stoichiometry.
Read More »