Mastering Stoichiometry: Your Ultimate Guide
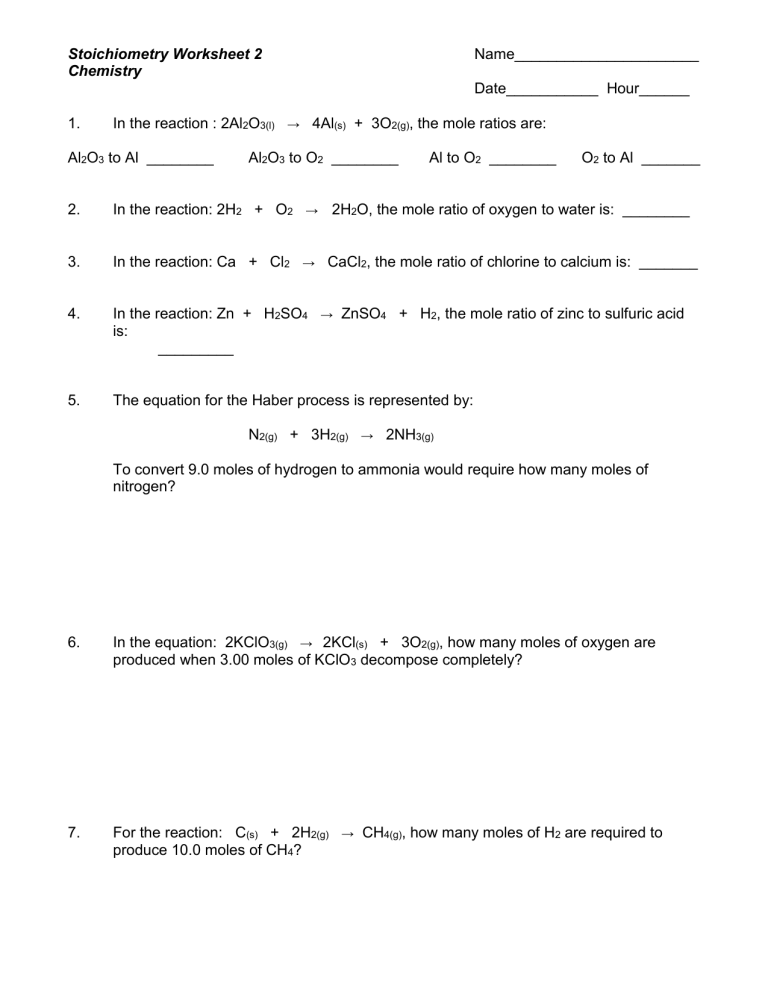
Understanding stoichiometry is crucial for anyone diving into the world of chemistry. This mathematical branch of chemistry not only helps in understanding the theoretical and experimental relationships between reactants and products in chemical reactions but also plays a pivotal role in various practical applications. Whether you're a student, a budding chemist, or someone involved in chemical processes, mastering stoichiometry can enhance your comprehension and execution of chemical reactions. Here, we provide an ultimate guide to mastering stoichiometry, ensuring that you can navigate through chemical calculations with confidence and ease.
The Basics of Stoichiometry

Stoichiometry revolves around the principle that elements combine in simple whole number ratios to form compounds. Here are the core concepts:
- Mole Concept: The foundation of stoichiometry, where one mole of any substance contains Avogadro’s number of particles (6.022 x 1023). Understanding moles helps quantify substances.
- Balanced Chemical Equations: These equations indicate the ratios of moles for reactants and products. They ensure mass conservation during chemical reactions.
- Molar Mass: The mass of one mole of a substance, which is crucial for stoichiometric calculations.
⚠️ Note: Always ensure your equations are balanced before proceeding with calculations to avoid errors.
Steps for Stoichiometric Calculations

Here’s a step-by-step guide to performing stoichiometric calculations:
- Write the Balanced Equation: This step cannot be overstated. Your equation needs to be correctly balanced.
- Identify Knowns and Unknowns: Determine what you know (e.g., the mass or volume of a reactant) and what you need to find out (e.g., the mass of a product).
- Convert Mass to Moles: Use the molar mass of known substances to convert mass to moles.
- Use Molar Ratios: Apply the mole ratio from the balanced equation to find the moles of the unknown substance.
- Convert Moles Back to Mass: Once moles of the unknown are known, convert this back to mass using molar mass.
- Check Your Calculations: Recheck your work, especially the stoichiometric coefficients.
Common Stoichiometric Calculations

Stoichiometry isn’t limited to just finding the mass of a product. Here are some common calculations:
- Limiting Reagent Calculation: Identify which reactant will run out first, limiting the amount of product that can be formed.
- Percent Yield: Compare actual yield with theoretical yield to find efficiency.
- Determining Reaction Extent: Use stoichiometry to determine how much of a reactant has reacted or how much product has been formed.
Applications of Stoichiometry

Stoichiometry is more than just a theoretical exercise:
- Industrial Chemistry: From fertilizers to pharmaceuticals, industries depend on stoichiometry to scale up production efficiently.
- Environmental Chemistry: To understand pollutant reactions, like the amount of gas produced in combustion.
- Biochemistry: To analyze reaction pathways and metabolic processes in living organisms.
- Food Science: For determining the nutritional composition of food and the reactions that occur during cooking.
Advanced Stoichiometry

As you dive deeper into chemistry, here are some advanced concepts:
- Complex Reaction Stoichiometry: Deals with multiple reactants or products in a reaction.
- Gas Phase Stoichiometry: Uses gas laws alongside chemical equations to calculate gas volumes or pressures.
- Solution Stoichiometry: Involves concentrations, often moles per liter (molarity), to calculate reaction outcomes in solutions.
In your journey to master stoichiometry, remember that practice is the key to proficiency. Here are some tips to help you along the way:
- Work with Real-life Examples: Apply stoichiometric calculations to real-world problems to gain practical insight.
- Use Software and Simulation Tools: These can help visualize and practice stoichiometric relationships dynamically.
- Engage in Collaborative Learning: Learning from peers or experts can provide new perspectives and enhance understanding.
- Embrace the Mole Concept: The mole is fundamental; grasp it thoroughly to simplify stoichiometric calculations.
💡 Note: Always double-check your work using dimensional analysis to ensure your units cancel correctly, leaving you with the desired unit.
By now, you should have a solid foundation in stoichiometry, understanding both its theoretical underpinnings and practical applications. The ability to calculate with confidence, interpret chemical equations, and apply stoichiometry to various chemical problems is not just about academic success but also about excelling in any field related to chemistry or its applications.
Remember, mastering stoichiometry means becoming adept at applying chemical principles to real-world scenarios, from controlling pollution to optimizing industrial processes, and even understanding biological processes. This guide has provided you with the tools and knowledge to delve deeper into the world of chemistry with greater confidence and skill. Keep practicing, keep learning, and soon, you'll find stoichiometry becoming second nature in your chemical explorations.
What is stoichiometry?

+
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It uses the principle that elements combine in simple whole number ratios to form compounds.
Why is understanding moles important in stoichiometry?

+
Moles are fundamental in stoichiometry because they allow chemists to work with a consistent unit of measure for the amount of substance, representing Avogadro’s number of particles. This enables the prediction of how much product can be formed from a given amount of reactant or vice versa.
How does stoichiometry apply to everyday life?

+
Everyday applications of stoichiometry include cooking (where recipes are often stoichiometric ratios), in environmental control to predict how pollutants interact, in healthcare for drug formulations, and in industry for optimizing chemical processes to minimize waste and cost.