Stoichiometry Made Simple: 5 Quick Worksheet Solutions

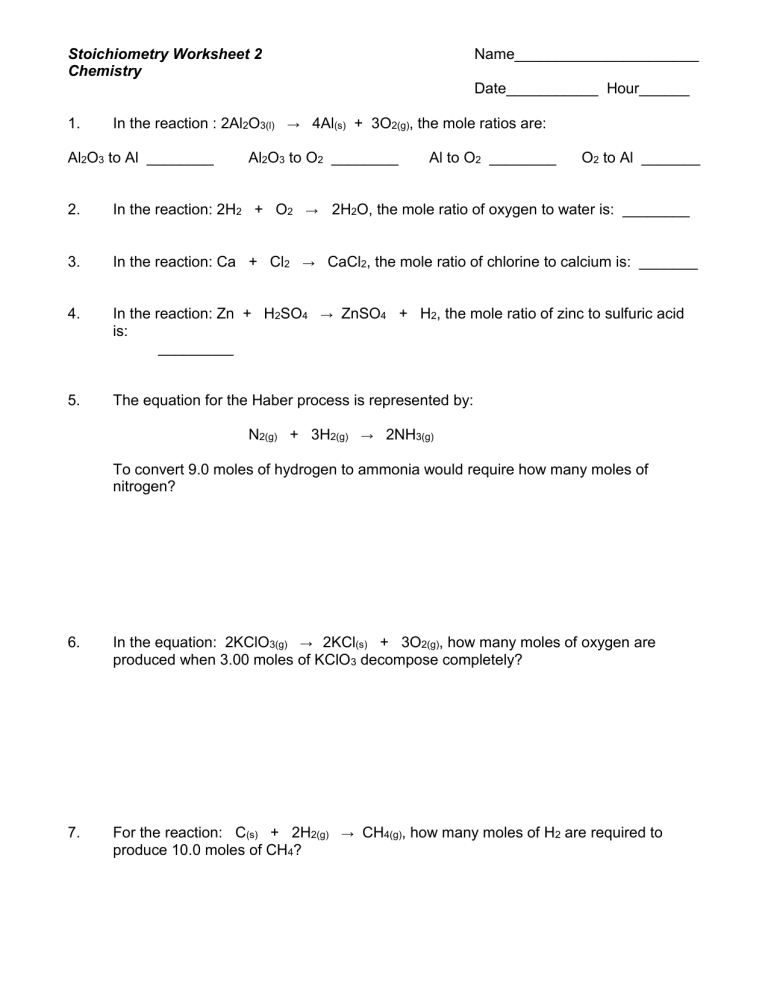
Stoichiometry is often considered one of the trickiest aspects of chemistry, but with the right approach, it can become one of the most straightforward. Today, we’re going to break down five common stoichiometry problems you might encounter on worksheets, providing you with clear, step-by-step solutions. This will not only help you solve these problems but also enhance your understanding of chemical reactions and their stoichiometric ratios.
Understanding Stoichiometry

Stoichiometry involves calculating the quantities of reactants and products in chemical reactions. Here’s a quick recap:
- The mole is a fundamental unit in stoichiometry, where 1 mole of a substance contains Avogadro’s number (6.022 × 1023) of entities.
- Stoichiometry uses balanced chemical equations to determine the ratios between reactants and products.
- Conversions between moles, mass, and volume are common in stoichiometric calculations.

Problem 1: Molar Ratio Calculation

Problem: Calculate the amount of oxygen (O2) needed to react completely with 2 moles of hydrogen (H2) in the formation of water (H2O).
- Write the balanced chemical equation:
2 H2 + O2 → 2 H2O - From the equation, we see that 2 moles of H2 react with 1 mole of O2.
- Since we have 2 moles of H2, we need:
1 mole O2
Solution: 1 mole of O2 is required.
Problem 2: Mass-Mole Conversion

Problem: How many grams of CO2 are produced when 5.00 moles of carbon © are burned?
- Write the balanced chemical equation:
C + O2 → CO2 - From the equation, 1 mole of C produces 1 mole of CO2.
- Molar mass of CO2 = 12 + (2 × 16) = 44 g/mol.
- For 5.00 moles of C, the mass of CO2 produced is:
5.00 mol × 44 g/mol = 220 g
Solution: 220 grams of CO2 are produced.
Problem 3: Limiting Reagent

Problem: You have 4.00 moles of Al and 6.50 moles of O2. What is the limiting reagent in the reaction forming Al2O3?
- Write the balanced chemical equation:
4 Al + 3 O2 → 2 Al2O3 - Calculate moles of Al2O3 if all Al or O2 were consumed:
- If Al limits:
4.00 mol Al × (2 mol Al2O3 / 4 mol Al) = 2.00 mol Al2O3 - If O2 limits:
6.50 mol O2 × (2 mol Al2O3 / 3 mol O2) = 4.33 mol Al2O3
- If Al limits:
- The lower value indicates Al is the limiting reagent.
Solution: Aluminum (Al) is the limiting reagent, producing 2 moles of Al2O3.
Problem 4: Yield Calculations

Problem: If the theoretical yield of NH3 is 10.00 grams from the reaction of nitrogen (N2) with hydrogen (H2), and the actual yield is 8.50 grams, what is the percent yield?
- Percent yield formula:
Percent Yield = (Actual Yield / Theoretical Yield) × 100% - Plugging in the values:
Percent Yield = (8.50 g / 10.00 g) × 100% = 85%
Solution: The percent yield is 85%.
Problem 5: Gas Volume Calculations
Problem: What volume of oxygen at STP is required to completely react with 1.00 gram of C3H8?
- Write the balanced chemical equation:
C3H8 + 5 O2 → 3 CO2 + 4 H2O - Convert mass of C3H8 to moles:
1.00 g / 44.10 g/mol = 0.0227 mol C3H8 - Find moles of O2 required:
0.0227 mol C3H8 × (5 mol O2 / 1 mol C3H8) = 0.1135 mol O2 - Convert moles of O2 to volume at STP (1 mol occupies 22.4 liters):
0.1135 mol × 22.4 L/mol = 2.55 L
Solution: 2.55 liters of oxygen are required.
💡 Note: Understanding the stoichiometric ratios and the conversions between different units is key to mastering these types of problems.
In summary, these five worksheet solutions demonstrate the practical application of stoichiometry in chemistry. Whether it's determining the limiting reagent, calculating theoretical and actual yields, or understanding the relationship between gas volumes and moles, stoichiometry remains an essential tool in predicting the outcomes of chemical reactions. By mastering these foundational concepts, you can approach even the most complex stoichiometric problems with confidence.
What is the significance of balanced equations in stoichiometry?

+
Balanced equations ensure that the law of conservation of mass is maintained, allowing us to calculate precise ratios of reactants and products. Without balance, stoichiometric calculations would be inaccurate.
Why is STP used in gas stoichiometry?

+
STP (Standard Temperature and Pressure) is used because it provides a common reference point for gas volume calculations, simplifying comparisons across different experiments or reactions.
Can you have a limiting reagent if one reactant is in excess?

+
Yes, the limiting reagent is the reactant that gets fully consumed, determining the maximum amount of product that can be produced. Excess reactants will remain unreacted after the reaction.