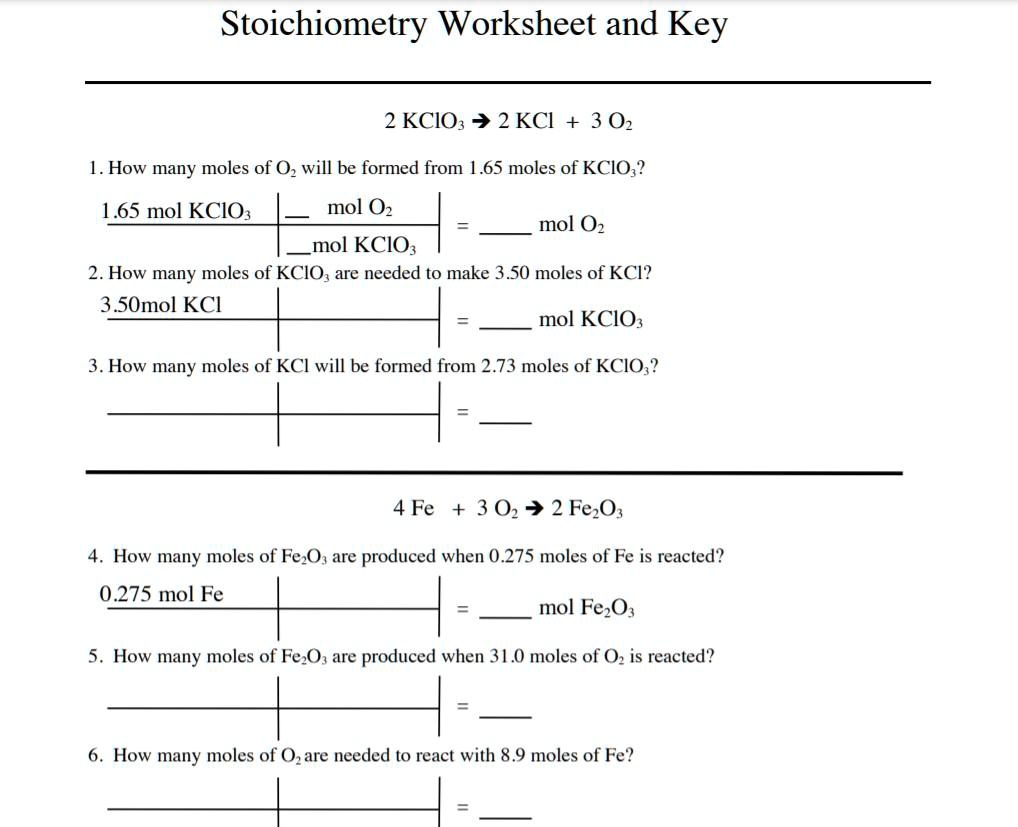
Stoichiometry Worksheet 2: Top Answers Revealed

Understanding Stoichiometry in Chemical Equations

Stoichiometry is a fundamental concept in chemistry, providing a framework for understanding the quantitative relationships between reactants and products in chemical reactions. As students and educators dive deeper into the intricacies of chemical reactions, mastering stoichiometry becomes an indispensable skill. This long-form blog post aims to elucidate Stoichiometry Worksheet 2 by revealing top answers, offering practical insights, and guiding students through the complexities of chemical stoichiometry.
Introduction to Stoichiometry

Before delving into specific problems from Stoichiometry Worksheet 2, let’s start with the basics:
- Balancing Chemical Equations: This is the cornerstone of stoichiometry, ensuring that the law of conservation of mass is upheld in a reaction.
- Mole Concept: Understanding the mole is vital as it acts as a bridge between the microscopic world of atoms and the macroscopic world of lab measurements.
- Molar Ratios: This is the heart of stoichiometry, showing the proportion of reactants and products involved in a reaction.
The Role of Stoichiometry in Problem Solving

Stoichiometry allows us to predict:
- The amount of product formed from given reactants
- The limiting reactant in a reaction
- Percent yield of a reaction
- Molarity in solution reactions
- Chemical reactions in industrial applications
Understanding these aspects is crucial for tackling the problems in Stoichiometry Worksheet 2.
Key Concepts in Stoichiometry

📝 Note: Always start by balancing the chemical equation before attempting to solve stoichiometric problems.
1. Converting Between Mass and Moles
In stoichiometry, you frequently need to convert the mass of a substance to moles or vice versa. This can be done using the molar mass of the substance, which is derived from the atomic masses listed on the periodic table.
2. Molar Ratios and Moles
The stoichiometric coefficient in a balanced chemical equation gives the molar ratio between reactants and products. This ratio is used to convert from moles of one substance to moles of another.
| Substance | Chemical Equation Coefficient | Molar Ratio |
|---|---|---|
| Reactant A | 2 | 2:1 |
| Product B | 1 | 1:2 |

3. Limiting Reagent
Determining the limiting reagent is essential in stoichiometry as it dictates how much product can actually be formed. It’s the reactant that is completely consumed when the reaction reaches completion.
📝 Note: The amount of product is always calculated based on the limiting reagent.
Solving Stoichiometry Worksheet 2

Now, let’s delve into Stoichiometry Worksheet 2 by breaking down and solving its problems.
Problem 1: Balanced Chemical Equation Conversion
Consider the following reaction: [ 2 Na + Cl_2 \rightarrow 2 NaCl ]
If you have 10 grams of sodium (Na) and 15 grams of chlorine gas (Cl_2), how many grams of sodium chloride (NaCl) can you produce?
To solve:
- Convert grams of Na and Cl_2 to moles using their molar masses.
- Use the molar ratios from the balanced equation to determine which reactant is limiting.
- Calculate the moles of NaCl produced from the limiting reagent.
- Convert moles of NaCl back to grams using its molar mass.
Problem 2: Molar Volume
What volume of H_2 gas would be produced at STP by reacting 25 grams of calcium hydride (CaH_2) with water? The balanced equation is: [ CaH_2 + 2 H_2O \rightarrow Ca(OH)_2 + 2 H_2 ]
- Determine moles of CaH_2 from the given mass.
- Use the stoichiometric ratio to find moles of H_2 gas produced.
- Convert moles of H_2 to liters using the fact that 1 mole of an ideal gas at STP occupies 22.4 liters.
Problem 3: Percent Yield
If 6 grams of sodium react with excess chlorine gas to produce sodium chloride, and you obtain 10 grams of NaCl, what is the percent yield?
- Calculate the theoretical yield using stoichiometry.
- Calculate the actual yield (given).
- Apply the percent yield formula: Percent Yield = (Actual Yield / Theoretical Yield) × 100%.
Summary and Insights

In this exploration of Stoichiometry Worksheet 2, we’ve seen how the principles of stoichiometry apply to real-world problem-solving in chemistry.
Key points to remember:
- Balancing chemical equations is the foundation for stoichiometric calculations.
- Understanding the mole concept allows for the conversion between mass and volume in reactions.
- Molar ratios from the balanced equation are crucial for determining quantities in reactions.
- The limiting reagent dictates the actual amount of product formed.
- Stoichiometry isn't just about calculations; it's about understanding the quantitative relationship between reactants and products, which is invaluable in industrial and laboratory settings.
📝 Note: Always double-check your units and conversions to avoid common errors in stoichiometric calculations.
Stoichiometry, while at first seeming complex, can be mastered with practice and understanding. Through Stoichiometry Worksheet 2, we’ve seen how these concepts come to life in real-world applications, from predicting product yields to understanding the efficiency of chemical reactions.
What is stoichiometry?

+
Stoichiometry is the study of quantitative relationships in chemical reactions, focusing on how elements or compounds react to form products.
Why is balancing chemical equations important?

+
Balancing chemical equations ensures the law of conservation of mass is followed, meaning the number of atoms in the reactants must equal those in the products.
How do you determine the limiting reagent in a reaction?

+
By calculating how much product each reactant can produce, the reactant that yields the least amount of product is the limiting reagent.
What is percent yield and why is it important?
+
Percent yield shows how efficiently a chemical reaction produces a product compared to the theoretical yield, which is important for assessing reaction conditions and efficiency.
Can stoichiometry be used outside the lab?

+
Yes, stoichiometry is crucial in industrial settings for determining optimal reaction conditions, yield predictions, and managing chemical processes economically.


