Solution Stoichiometry Worksheet: Master with Answers

In the realm of chemistry, one concept that serves as the cornerstone for understanding chemical reactions is stoichiometry. This term refers to the quantitative relationships between reactants and products in a chemical reaction. Today, we'll delve into solution stoichiometry—a subset of stoichiometry that deals specifically with reactions in solution. Whether you're a student grappling with chemistry coursework or an enthusiast seeking a deeper understanding of chemical calculations, this blog post will serve as your comprehensive guide to mastering solution stoichiometry with answers to common problems.
Understanding Solution Stoichiometry

Solution stoichiometry involves calculating the quantities of substances in a chemical reaction that occur when they are dissolved in a liquid, usually water. The key to mastering solution stoichiometry is understanding how to relate the volume and concentration of solutions to moles of substances, which then allows for stoichiometric calculations.
- Molarity (M): It's the concentration of a solution expressed as the number of moles of solute per liter of solution. The equation for molarity is:
M = n/V, where:- M = molarity (mol/L)
- n = moles of solute
- V = volume of solution (in liters)
- Volume: The space occupied by a substance, often given in liters (L) or milliliters (mL).
- Number of moles: This is calculated using the formula:
n = m/M, where:- n = moles
- m = mass of solute (in grams)
- M = molar mass of solute
Steps to Solving Solution Stoichiometry Problems

1. Identify and Write the Balanced Chemical Equation

First, you need a chemical equation to know how substances react. For example, consider the reaction:
NaOH + HCl → NaCl + H2O
Ensure that the equation is balanced, indicating the correct stoichiometric coefficients for reactants and products.
2. Determine the Molarity or Molar Mass

From the given information, find the molarity of the solutions involved in the reaction or calculate the molar mass if dealing with solids.
| Substance | Molar Mass (g/mol) |
|---|---|
| NaOH | 40 |
| HCl | 36.5 |
| NaCl | 58.5 |

3. Calculate the Moles of Known Substance

Using the formula n = M × V, calculate the moles of a substance you know the volume and concentration of.
⚠️ Note: Ensure volumes are in liters when using this formula.
4. Use the Balanced Equation to Find Moles of Unknown

Use the stoichiometry of the reaction to find moles of the unknown substance. For example, if you know the moles of HCl used, the moles of NaOH required will be the same due to the 1:1 ratio in the balanced equation.
5. Calculate Concentration or Volume of Unknown

If you’re looking for concentration:
M = n/V
If you’re looking for volume:
V = n/M
Common Problems and Solutions in Solution Stoichiometry

Problem 1: Calculating Moles Given Volume and Molarity
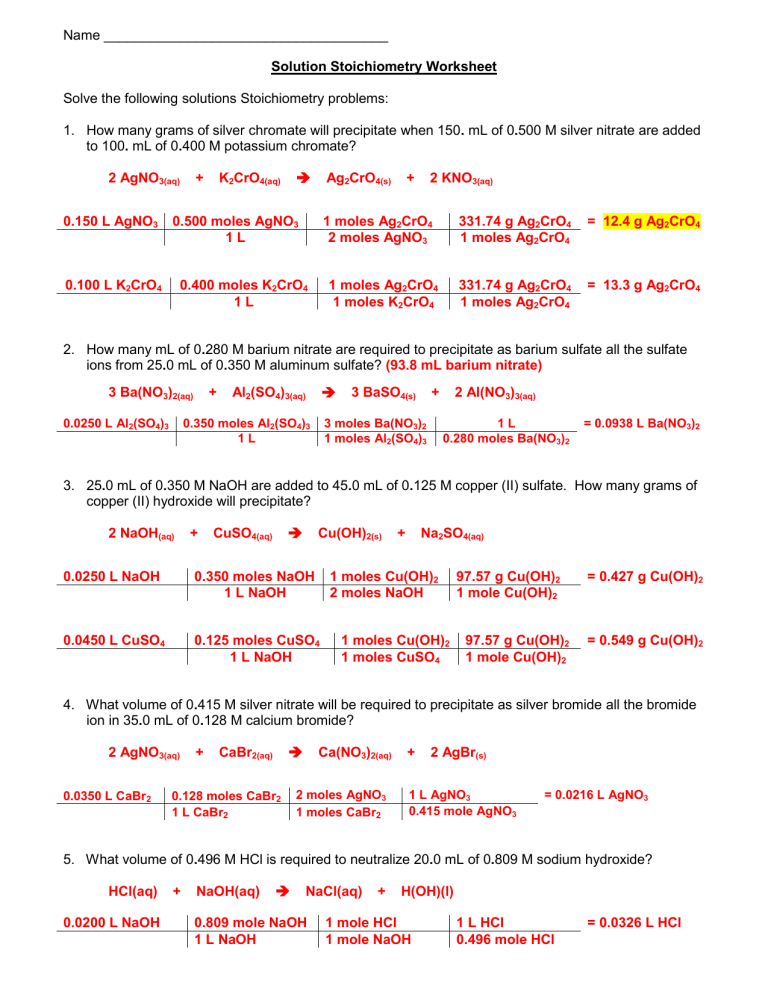
Given 25 mL of a 0.5 M HCl solution, how many moles of HCl are present?
n = M × V = 0.5 mol/L × 0.025 L = 0.0125 mol
Problem 2: Finding Volume with Known Moles and Concentration

How much volume of a 0.25 M NaOH solution is needed to completely react with 0.0125 moles of HCl?
V = n/M = 0.0125 mol / 0.25 mol/L = 0.05 L or 50 mL
Problem 3: Determining Molarity from Mass of Solute and Volume

If you dissolve 2.0 grams of NaOH in water to make 100 mL solution, what is the molarity?
n = m/M = 2.0 g / 40 g/mol = 0.05 mol
M = n/V = 0.05 mol / 0.1 L = 0.5 M
Practical Applications

Solution stoichiometry isn’t just an academic exercise; it has numerous practical applications:
- Pharmaceutical Industry: For creating medications with specific concentrations.
- Environmental Science: For analyzing pollutants in water samples.
- Food Science: For understanding nutritional content or creating specific food products.
- Industrial Processes: For controlling chemical reactions in manufacturing.
Conclusion

The art of mastering solution stoichiometry involves understanding the relationship between the amounts of reactants and products in a chemical reaction. By learning to manipulate the molarity, volume, and moles, you can unlock the quantitative aspects of chemistry, making both theoretical and practical applications more accessible. This guide should serve as your starting point to tackle solution stoichiometry problems with confidence, understanding not just the how but the why behind each calculation. Remember, consistent practice with stoichiometry worksheets and real-life applications will solidify your skills and deepen your appreciation for the fascinating precision of chemical reactions.
What is molarity and why is it important in solution stoichiometry?

+
Molarity (M) is a measure of the concentration of a solute in a solution, expressed as the number of moles of solute per liter of solution. It’s crucial because it allows chemists to know how much of a particular substance is present in a solution, which is essential for predicting how much of another substance will be needed or produced in a reaction.
Can I use solution stoichiometry for reactions that do not occur in water?

+
Yes, while water is commonly used as a solvent, solution stoichiometry can be applied to reactions in any solvent where the concentration can be expressed as moles per liter. The principles remain the same; only the solvent might change.
How does temperature affect stoichiometric calculations?
+
Temperature primarily affects the solubility of the solutes and the volume of the solutions due to thermal expansion or contraction. While the stoichiometry itself is not altered, the molarity of the solution might change if the volume changes, so temperature is a factor to consider when doing solution stoichiometry.



