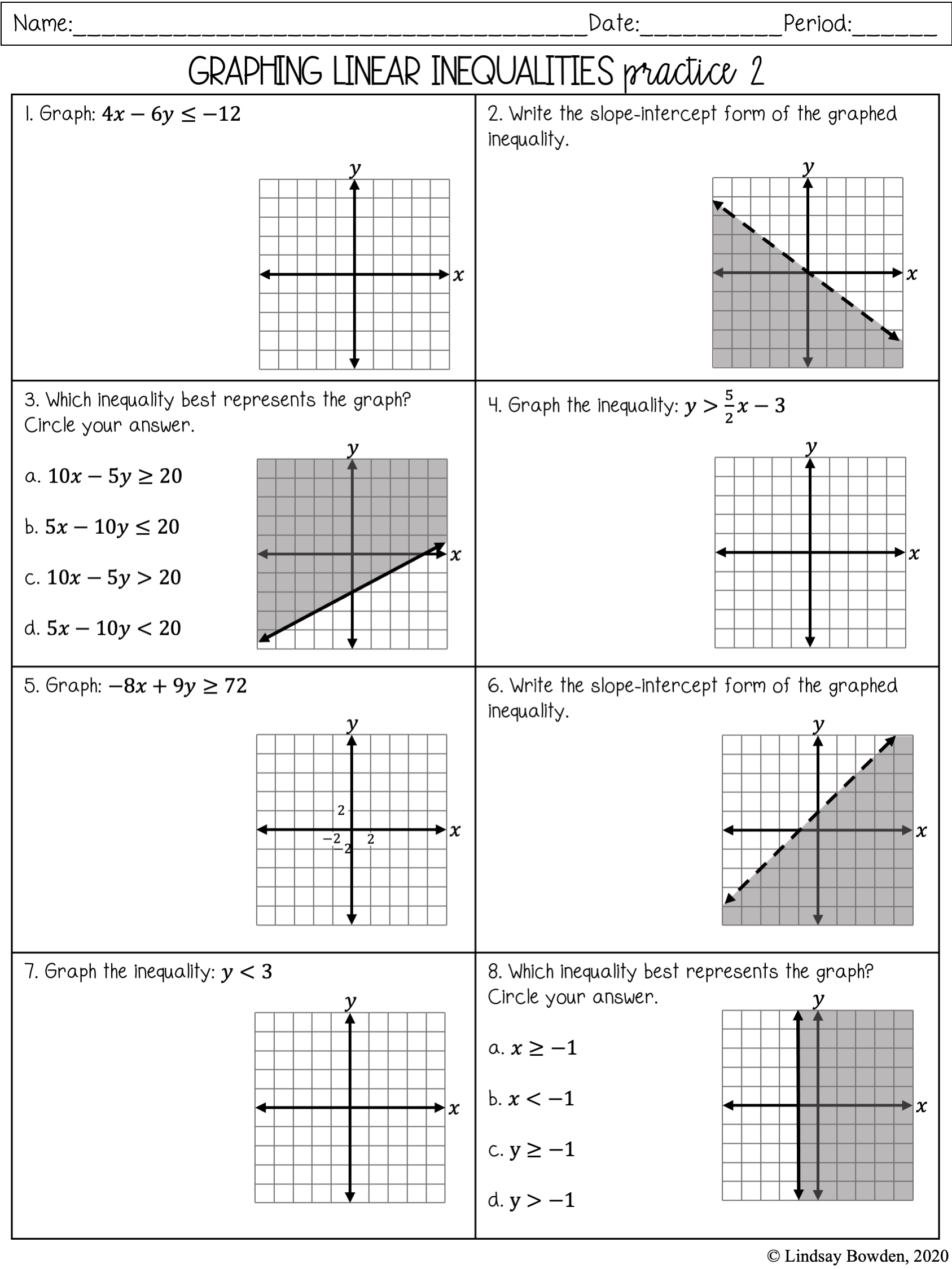
5 Proven Answers for Mole-Mole Stoichiometry Problems
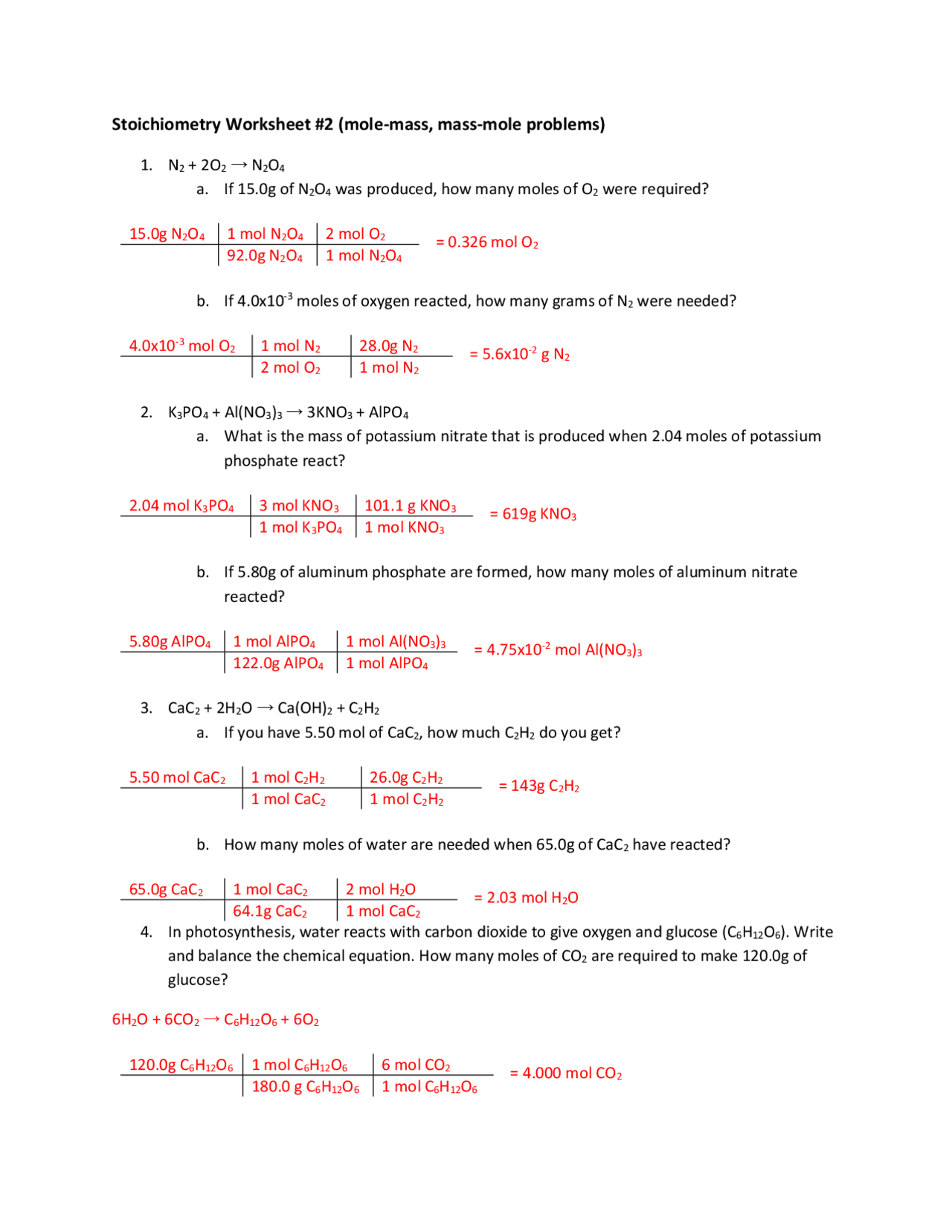
Delving into the realm of chemistry, one finds themselves entangled with various forms of calculations, including mole-mole stoichiometry. Understanding this foundational concept in chemistry is not only essential for academic success but also critical in real-world applications like pharmaceuticals, environmental science, and material engineering. This post will guide you through five proven answers for solving mole-mole stoichiometry problems, ensuring you grasp this topic comprehensively.
Understanding Mole-Mole Stoichiometry

Mole-mole stoichiometry involves the study of the relationship between the amount of reactants consumed and the amount of products produced in a chemical reaction, expressed in moles. Here's how you can approach these problems:
- Identify the balanced chemical equation.
- Determine the mole ratio from the coefficients of the equation.
- Use the mole ratio to convert from one substance to another.
Step-by-Step Guide to Solving Mole-Mole Problems

Let's explore the step-by-step process:
- Write the Balanced Equation: Always begin with a balanced chemical equation. This ensures the stoichiometric ratios are accurate.
- Identify Known and Unknown: Determine which substances you have quantities for and which you need to find.
- Set Up the Conversion: Use the mole ratio from the balanced equation to set up your conversion.
- Calculate: Perform the calculation to find the moles of the unknown substance.
- Double-Check Your Work: Ensure your results make sense with the law of conservation of mass.
Example Problem 1: Simple Mole-Mole Conversion

Let's solve a basic problem where 3 moles of nitrogen gas (N2) react with hydrogen gas (H2) to form ammonia (NH3):
💡 Note: The balanced equation for this reaction is N2 + 3H2 → 2NH3.
Given 3 moles of N2, how many moles of NH3 can be produced?
| Compound | Moles | Mole Ratio (N2 : NH3) | Moles of NH3 |
|---|---|---|---|
| N2 | 3 | 1 : 2 | 6 |

Using the mole ratio from the balanced equation:
3 moles N2 × (2 moles NH3 / 1 mole N2) = 6 moles NH3
Example Problem 2: Limiting Reactant Scenario

Now let's tackle a problem involving a limiting reactant. Consider the reaction between aluminium (Al) and oxygen (O2) to form aluminium oxide (Al2O3):
💡 Note: The balanced equation here is 4Al + 3O2 → 2Al2O3.
If we have 10 moles of Al and 12 moles of O2, what is the maximum amount of Al2O3 that can be formed?
First, we calculate the moles of Al2O3 if all of Al or O2 were consumed:
10 moles Al × (2 moles Al2O3 / 4 moles Al) = 5 moles Al2O3 12 moles O2 × (2 moles Al2O3 / 3 moles O2) = 8 moles Al2O3
Since Al produces less Al2O3, it's the limiting reactant, and the maximum yield is:
5 moles Al2O3
Example Problem 3: Percentage Yield Calculation

This problem involves calculating the percentage yield, a crucial metric in industrial applications. Given the previous reaction where 5 moles of Al produced 4 moles of Al2O3 (instead of the expected 5), what's the percentage yield?
Percentage Yield = (Actual Yield / Theoretical Yield) × 100% Actual Yield = 4 moles Al2O3, Theoretical Yield = 5 moles Al2O3 Percentage Yield = (4 / 5) × 100% = 80%
Example Problem 4: Mass-Mole Conversion

In this scenario, we'll convert from the mass of a reactant to the moles of a product. Let's use the reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H2O):
💡 Note: The balanced equation is HCl + NaOH → NaCl + H2O.
If 13.5 grams of NaOH reacts with HCl, how many moles of H2O are produced?
Moles of NaOH = Mass / Molar Mass = 13.5 g / (40.0 g/mol) = 0.3375 moles Since the reaction is 1:1, 0.3375 moles of NaOH produces 0.3375 moles of H2O.
Example Problem 5: Complex Stoichiometry

Finally, let's tackle a more complex problem where you need to find the moles of one substance given an unconventional starting point:
Given a chemical reaction where glucose (C6H12O6) reacts with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O) according to the equation:
💡 Note: The balanced equation is C6H12O6 + 6O2 → 6CO2 + 6H2O.
If the pressure of CO2 produced at a certain temperature and volume is given, you can use the Ideal Gas Law (PV = nRT) to convert this to moles of CO2, then use the stoichiometry to find the moles of glucose needed:
The endgame of this journey through mole-mole stoichiometry is not just the mastery of problem-solving but the understanding of how chemical reactions underpin the very fabric of our physical universe. From determining the theoretical yield to managing complex industrial processes, mole-mole stoichiometry serves as the key to unlocking the potential of chemical transformations. This knowledge, once integrated into practice, empowers scientists and engineers to navigate the complexities of reactions with precision and foresight, turning theoretical possibilities into tangible outcomes.
What is stoichiometry?
+
Stoichiometry is the calculation of quantitative relationships between the reactants and products in chemical reactions. It uses the mole concept and involves mole-mole relationships.
Why is the balanced chemical equation important in stoichiometry?

+
A balanced chemical equation is essential because it shows the relative quantities of reactants and products, which are necessary to calculate the amount of substances involved in the reaction.
What is the limiting reactant in a chemical reaction?

+
The limiting reactant is the substance in a chemical reaction that is entirely consumed first, which limits the amount of product that can be formed. It determines the maximum amount of product possible.