5 Tips for Calculating Percent Yield Easily

Calculating percent yield is a crucial skill in chemistry, whether you're a student or a professional chemist. It allows you to assess the efficiency of chemical reactions by comparing the actual yield of a product to its theoretical yield. Here are some effective tips that can help streamline this calculation:
1. Understand the Basics of Percent Yield Calculation


Before diving into the calculation, ensure you understand the fundamental concepts:
- Actual Yield: The amount of product obtained in reality.
- Theoretical Yield: The maximum amount of product that can theoretically be produced from given amounts of reactants.
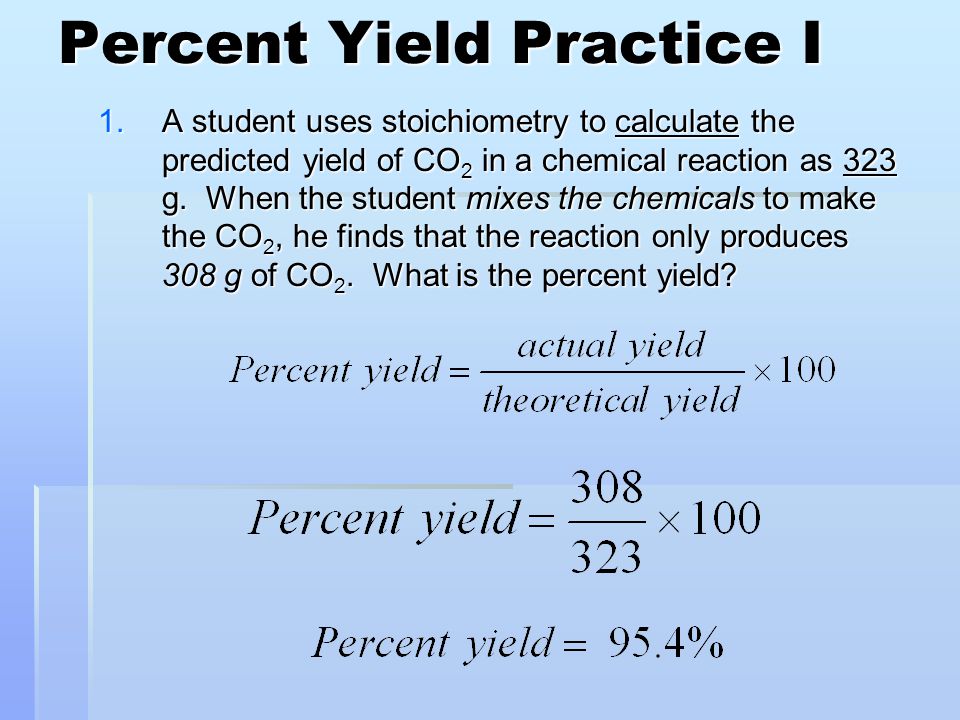
- Percent Yield: Calculated as the ratio of actual yield to theoretical yield, expressed as a percentage.
The formula for percent yield is:
Percent Yield = (Actual Yield / Theoretical Yield) * 100%
2. Meticulously Record All Data

Your calculations are only as good as the data you input:
- Use precision balances and accurate measuring tools for reactants and products.
- Note down the exact masses or volumes of substances before and after reactions.
- Keep a detailed lab notebook to refer back to when performing calculations.
🔍 Note: Double-check your measurements for accuracy, as small errors can significantly skew your yield calculations.
3. Use Dimensional Analysis

Dimensional analysis, or factor-label method, can simplify complex calculations:
- Set up equations where units cancel out, ensuring correct conversion.
- For example, if you need to convert moles to grams, use molar masses as conversion factors.
- Work step-by-step to minimize mistakes in calculations.
4. Apply Stoichiometry Correctly

Stoichiometry is key to determining theoretical yield:
- Understand the balanced chemical equation for the reaction.
- Use stoichiometric ratios to calculate how much product should form from given amounts of reactants.
- Consider the limiting reagent, which will dictate the amount of product possible in practice.
| Reaction Step | Description |
|---|---|
| Balancing Equation | Ensure the equation is balanced for atom conservation. |
| Find Limiting Reagent | Determine which reactant is in excess or limiting. |
| Theoretical Yield Calculation | Use stoichiometry to calculate the theoretical product yield. |

5. Round Off At the Last Step

Avoid intermediate rounding:
- Perform all calculations with exact figures or to a high degree of precision.
- Only round off your final percentage yield to the correct number of significant figures.
- This minimizes the compounding of rounding errors in the calculation process.
In conclusion, calculating percent yield isn't just about plugging numbers into an equation. It involves understanding the chemistry behind the process, meticulous data collection, correct application of stoichiometry, and careful calculation techniques. By following these five tips, you'll be able to calculate percent yield more easily, reducing the chances of mistakes and obtaining a more accurate understanding of your reaction's efficiency.
Why is percent yield less than 100%?
+
Percent yield is often less than 100% due to factors like incomplete reactions, side reactions, loss during transfer, or experimental errors. These are inherent in any laboratory setting.
What does a high percent yield indicate?

+
A high percent yield indicates that the reaction has been efficient, with minimal loss of reactants to side reactions or inefficiencies in the experimental setup.
How can I improve my percent yield?

+
To improve percent yield, you can optimize reaction conditions, minimize losses, use purer starting materials, or adjust the reaction time and temperature to favor the formation of the desired product.