Chemistry Word Equations Worksheet: Simplified Learning

In the intricate world of chemistry, understanding reactions can be a daunting task for beginners. Yet, there is an effective tool for simplifying this challenge: word equations. These equations translate chemical reactions into plain language, making it easier to grasp what's happening at the molecular level. This blog post delves into the mechanics of word equations, guiding students and enthusiasts through the process of mastering chemistry with an approachable method.
What Are Word Equations?

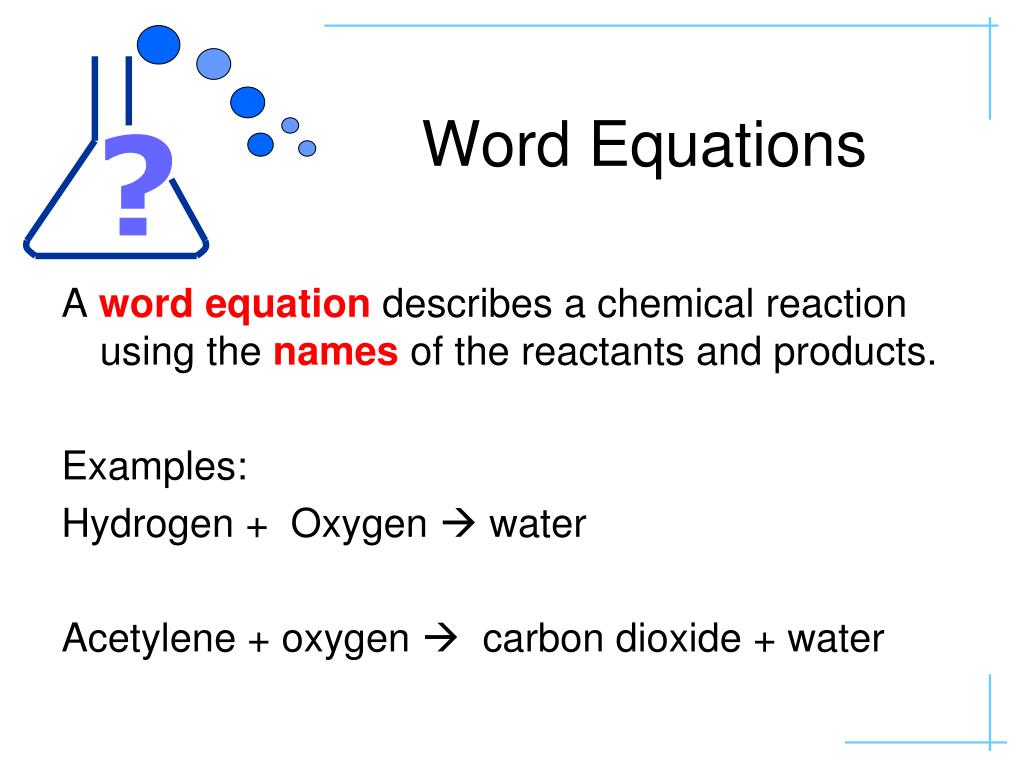
Word equations are chemical equations expressed in words instead of symbols. They provide a linguistic description of a reaction, detailing reactants, products, and often the state (such as solid, liquid, gas, or aqueous) of each component involved in the reaction. This approach is particularly helpful for those new to chemistry because it removes the initial barrier of understanding chemical symbols and formulas.
How to Write a Word Equation

Here’s a step-by-step guide to writing effective word equations:
- Identify the reactants and products: Begin by noting down the chemicals or substances involved in the reaction, separating them into reactants (what you start with) and products (what you end with).
- Note the states: If necessary, add the state symbols or conditions after each component. Use abbreviations like (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous solution.
- Write out the equation: Construct the equation by placing reactants on the left, an arrow pointing to the right indicating “reacts to form” or “yields,” followed by the products. For instance:
- Hydrogen + Oxygen → Water
- Balance the equation: While word equations might not require balancing, ensuring the mass conservation principle by balancing symbols (if necessary) can help in translating the word equation to a chemical one later on.
🔬 Note: While word equations provide a basic understanding, they often do not capture the complexity of chemical reactions like balanced chemical equations do, so consider this as a stepping stone to more detailed chemical notation.
Practical Examples of Word Equations
Let’s look at some real-life chemical reactions expressed as word equations:
| Reaction | Word Equation |
|---|---|
| Photosynthesis | Carbon dioxide + Water + Light Energy → Glucose + Oxygen |
| Combustion of Methane | Methane + Oxygen → Carbon Dioxide + Water Vapor |
| Neutralization | Sodium Hydroxide + Hydrochloric Acid → Sodium Chloride + Water |

These examples show how various types of reactions, from biological processes to common household reactions, can be described using word equations.
Why Use Word Equations?

The beauty of word equations lies in their accessibility:
- They serve as a bridge to chemical notation: For students struggling with chemical formulas, word equations provide an easier way to understand the basics of chemical reactions before diving into the more complex symbolic notations.
- Clarity in understanding: They articulate the reaction in everyday language, which can reduce confusion and make complex ideas more digestible.
- Enhanced learning: By first understanding the reaction in words, students can then transition to symbols with greater confidence and comprehension.
Word Equations vs. Chemical Equations

Understanding the difference between word equations and chemical equations is vital:
| Aspect | Word Equations | Chemical Equations |
|---|---|---|
| Format | Use words to describe reactants and products | Use chemical formulas and symbols |
| Balancing | Not strictly necessary | Must be balanced to follow the law of conservation of mass |
| Complexity | Less complex, more intuitive | More detailed, requires understanding of symbols |
| Use | Primarily educational, introductory | Professional, scientific, academic use |
Tips for Learning with Word Equations

Here are some strategies to maximize learning through word equations:
- Practice writing equations: Convert everyday reactions you observe or study into word equations to enhance retention and understanding.
- Relate to everyday life: Find examples of reactions in your daily life or the environment to make learning more tangible.
- Move to symbols: Once you’re comfortable with word equations, start translating them into chemical equations for a deeper understanding.
💡 Note: Regular practice is key to mastering word equations. Over time, you'll find it easier to switch between word and chemical equations seamlessly.
Conclusion

The use of word equations in chemistry education represents a thoughtful approach to understanding the reactions that shape our world. They bridge the gap between everyday language and scientific notation, providing a stepping stone for deeper learning. Through this method, students can not only grasp the essence of chemical reactions but also build confidence in tackling more complex chemical concepts. As you progress in your study of chemistry, remember that word equations serve as a valuable tool, making the subject more approachable and engaging. Keep exploring, practicing, and converting reactions into words, and soon, the symbolic language of chemistry will become second nature.
Can I write all chemical reactions as word equations?

+
Yes, theoretically you can describe any chemical reaction with a word equation, though complex reactions might be challenging to express concisely.
Are word equations less accurate than chemical equations?

+
They are less precise in terms of stoichiometry and chemical detail but are accurate in conveying the basic premise of a reaction.
How can I practice with word equations?

+
Look for everyday reactions, like cooking or cleaning, and describe them using word equations. Also, practice converting given chemical equations to word equations.
Do word equations help with exam preparation?

+
Yes, they can be incredibly helpful for understanding the core concepts of reactions, making it easier to answer descriptive questions in exams.



