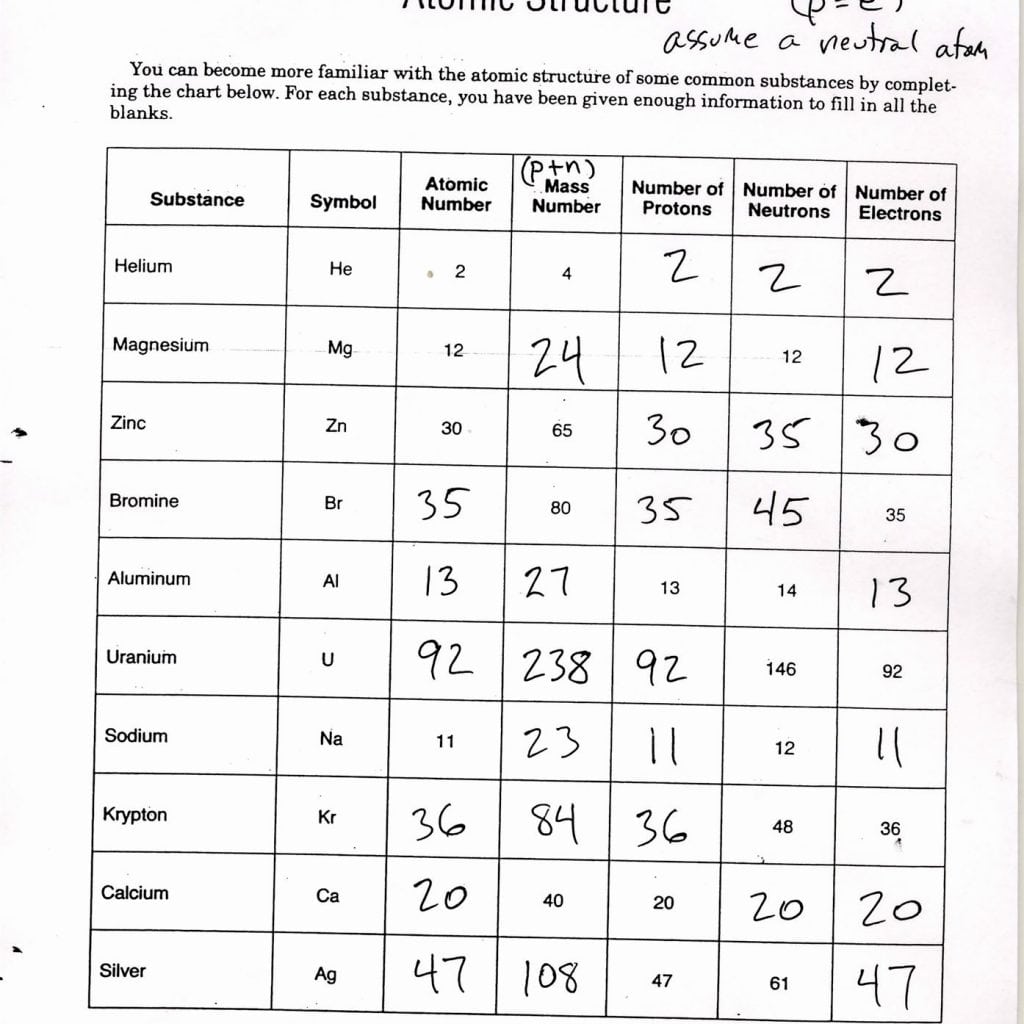
7 Ways to Master Specific Heat Calculations

In thermodynamics, the specific heat of a substance is a fundamental property that helps in understanding how much heat energy is required to change its temperature. This blog post will guide you through seven key methods and tips to master calculations involving specific heat. These methods not only help with academic tasks but are also crucial for practical applications in science, engineering, and even cooking.
Understanding Specific Heat


Before diving into calculations, it’s essential to grasp what specific heat means. Specific heat capacity is the amount of heat per unit mass needed to raise the temperature by one degree Celsius. It is denoted by c and has units of joules per gram per degree Celsius (J/g°C).
1. Memorize Common Specific Heats

- Water: 4.184 J/g°C
- Ice: 2.09 J/g°C
- Aluminum: 0.900 J/g°C
- Iron: 0.450 J/g°C
Knowing these values by heart will significantly speed up your calculations, especially in exams or when you need to perform quick assessments.
2. Master the Heat Transfer Equation

The equation for heat transfer involving specific heat is:
Q = m * c * ΔT
Where:
- Q is the heat energy in joules (J)
- m is the mass in grams (g)
- c is the specific heat capacity (J/g°C)
- ΔT is the change in temperature (Tfinal - Tinitial)
3. Apply Dimensional Analysis

Make sure your units are consistent. If you’re mixing grams with kilograms, convert all quantities to the same unit before calculating.
🔎 Note: Always ensure that your units cancel out correctly to end with joules for Q.
4. Practice with Simple Scenarios

Begin with simple examples like:
- How much energy is needed to heat 100g of water from 20°C to 80°C?
- What is the final temperature if 150J of heat is added to 50g of aluminum at 25°C?
These scenarios help in understanding the application of the equation in real-life situations.
5. Use Calorimetry for Complex Systems

Calorimetry involves measuring changes in heat content by mixing substances of known specific heats. Here’s a table to illustrate how you might set up such an experiment:
| Substance | Mass (g) | Initial Temperature (°C) | Final Temperature (°C) | Specific Heat (J/g°C) |
|---|---|---|---|---|
| Water | 100 | 20 | ? | 4.184 |
| Aluminum | 50 | 80 | ? | 0.900 |
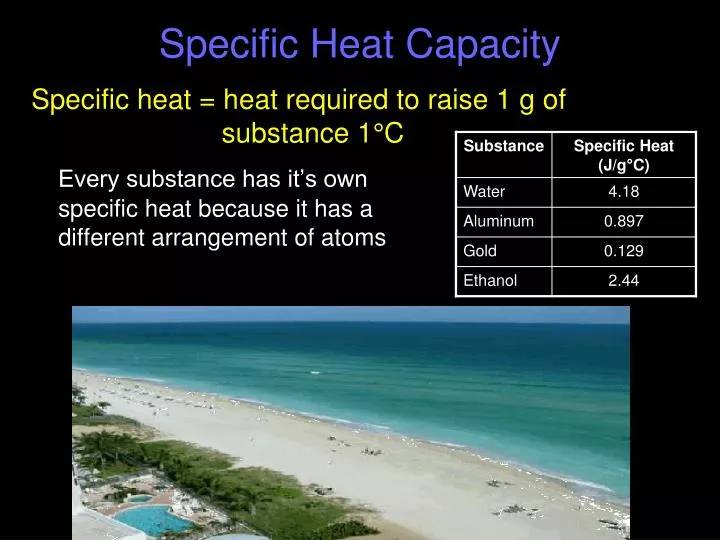
Using calorimetry, you can find the final temperature of the system or the heat exchanged.
6. Account for Phase Changes
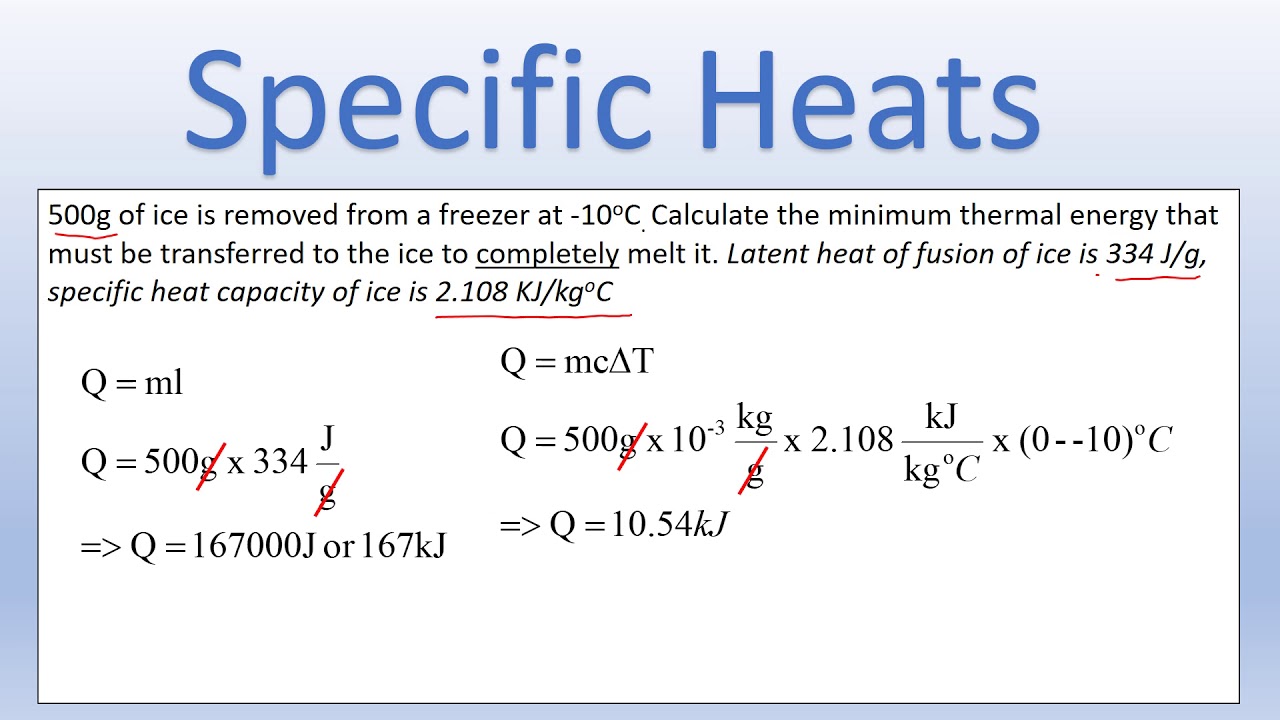
When a substance changes phase (e.g., from liquid to gas), specific heat calculations need to include latent heat:
- Calculate heat for temperature change:
Qtemp = m * c * ΔT
Qlatent = m * L
⚠️ Note: Specific heat values are valid only for temperature changes within one phase.
7. Use Temperature-Volume Graphs

For an advanced understanding, temperature-volume graphs can help visualize how heat affects substances:
- The slope of the line gives the heat capacity.
- Flat lines indicate phase transitions.
To recap, mastering specific heat calculations involves:
- Memorizing common specific heats for efficiency.
- Understanding and correctly applying the heat transfer equation.
- Converting units properly through dimensional analysis.
- Practicing with both simple and complex scenarios.
- Using calorimetry for systems with multiple substances.
- Accounting for latent heat during phase changes.
- Visualizing heat effects with temperature-volume graphs.
Why is specific heat important in cooking?

+
Specific heat helps cooks determine how long foods need to cook. For example, knowing the specific heat of water helps in adjusting cooking times for boiling or poaching, ensuring food is cooked thoroughly without overcooking.
Can specific heat change with temperature?

+
Yes, specific heat can vary with temperature. For many substances, the specific heat capacity changes slightly with temperature, which is why tables for specific heat often list values at specific temperatures.
How do phase changes affect specific heat calculations?

+
During phase changes, heat is absorbed or released without a change in temperature, so specific heat is not directly applicable. Instead, you calculate using latent heat, which accounts for the energy needed to change the phase of the substance.
Is there a simple way to measure specific heat at home?
+While not perfectly accurate, you can estimate specific heat by heating water and observing the temperature change of an object placed in it. By measuring the change in temperature and knowing the mass and specific heat of water, you can work backwards to find the object’s specific heat.
By following these methods, you’ll enhance your ability to deal with heat transfer in both theoretical and practical contexts, from engineering applications to everyday cooking, ensuring you’re well-equipped to handle any problem involving specific heat calculations.