Ionic Compounds Naming Worksheet Answers: Simplified Guide

Understanding the naming system for ionic compounds can be quite a challenge, but with a simplified guide, it becomes far easier to grasp. Whether you're a student embarking on your journey through chemistry, or just someone with a curious mind, knowing how to name these compounds is key to understanding their properties and reactions.
Introduction to Ionic Compounds

Ionic compounds are formed through the transfer of electrons, creating positively and negatively charged ions. These compounds are typically formed between metals, which lose electrons to become positive cations, and non-metals, which gain those electrons to form negative anions. Here, we will break down the naming process into simple steps:
1. Identifying the Cations and Anions

Begin by identifying the metal (cation) and the non-metal (anion) in your compound. Here’s how you can recognize them:
- Cations: Metals are usually on the left side of the periodic table or are transition metals.
- Anions: Non-metals are on the right side or are from groups 15, 16, and 17.
2. Using the Periodic Table

The periodic table is a crucial tool for naming. For main group elements:
- Group 1A and 2A metals: Always have a fixed charge. Sodium (Na+) or Magnesium (Mg2+) ions are good examples.
- Non-metals: Their charges are predictable based on their group number (e.g., Oxygen forms O2- from group 6A).
3. Nomenclature of Simple Ionic Compounds
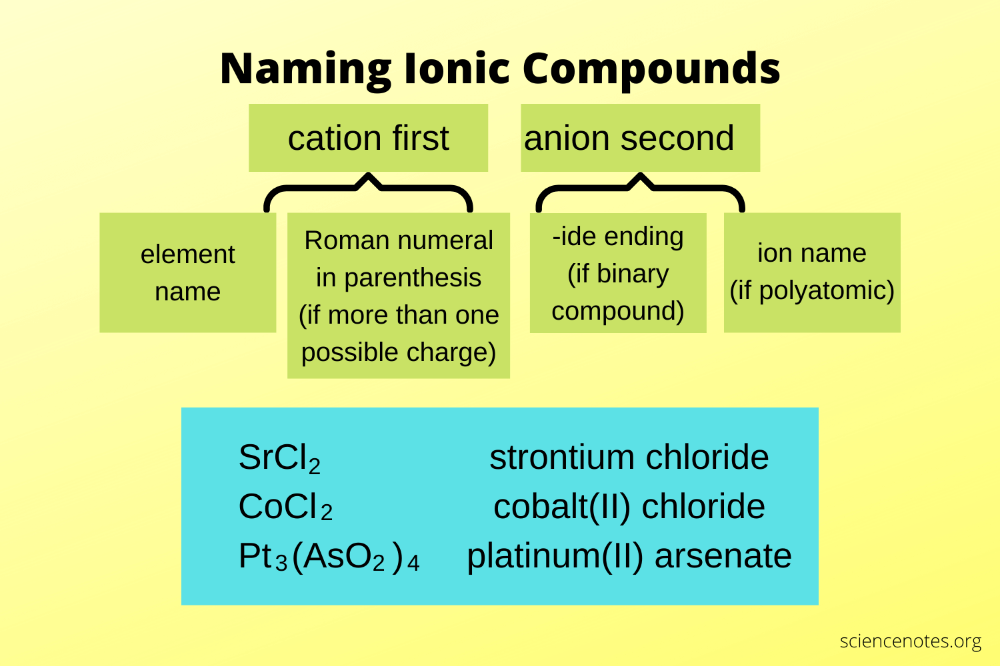
Now, let’s dive into the naming:
- Metal + Non-metal: Simply name the metal followed by the non-metal with an -ide ending.
- Example: NaF becomes sodium fluoride.
4. Compounds with Variable-Charge Metals

Transition metals and some metals like tin and lead can have variable charges:
- Roman Numerals: Use Roman numerals in parentheses after the metal’s name to indicate its charge.
- Example: FeCl2 and FeCl3 would be iron(II) chloride and iron(III) chloride respectively.
5. Polyatomic Ions

When dealing with polyatomic ions:
- Polyatomic ion ending in -ate or -ite: You can name these ions by keeping the suffix as is.
- Example: NO3- is nitrate, and NO2- is nitrite.
6. Handling Hydrates

Some compounds include water molecules in their structure, known as hydrates:
- Indicate the number of water molecules using prefixes like mono-, di-, tri- and then “hydrate.”
- Example: CuSO4·5H2O would be copper(II) sulfate pentahydrate.
7. Naming Binary Covalent Compounds

Binary covalent compounds use prefixes to indicate the number of atoms of each element:
- 1: mono-, 2: di-, 3: tri-, 4: tetra-, etc.
- The element named first gets the lowest number of atoms.
- Example: CO2 is carbon dioxide, and PCl5 is phosphorus pentachloride.
🌟 Note: When the compound includes hydrogen, it usually starts the name when it's not bonded with metal (e.g., HCl - hydrogen chloride).
Worksheet Practice and Answers

Here is a sample worksheet with answers to help you practice:
| Chemical Formula | Compound Name |
|---|---|
| KCl | Potassium chloride |
| MgO | Magnesium oxide |
| Fe2O3 | Iron(III) oxide |
| CuSO4 | Copper(II) sulfate |
| CO | Carbon monoxide |

📝 Note: Transition metals like iron can exhibit multiple charges, hence the use of Roman numerals in their names.
🔍 Note: Hydrates require an additional step to count the water molecules and add the proper prefix.
Understanding the naming of ionic compounds is fundamental for chemistry students. This guide has demystified the process, breaking it down into manageable steps:
- Recognizing cations and anions
- Using the periodic table to determine charges
- Naming simple compounds
- Dealing with variable charges
- Handling polyatomic ions
- Naming hydrates
- Naming covalent compounds
By following these steps and practicing with the worksheet provided, you'll become adept at naming ionic compounds. Remember, consistency in practice will lead to mastery. Keep referencing the periodic table and the ion tables for guidance, and soon you'll be naming compounds with ease.
How do I know when to use Roman numerals in naming ionic compounds?

+
You use Roman numerals when a metal can have more than one charge. Transition metals like copper, iron, and metals from the ‘tin and lead’ group often have variable charges. The Roman numeral indicates the specific charge of the metal cation in the compound.
Can non-metals form cations?

+
Under specific conditions, like in some organometallic compounds or in non-aqueous solvents, non-metals can indeed form positive ions, but this is an exception rather than the rule.
How do I name an ionic compound with a polyatomic ion?

+
Name the metal or ammonium (if present) first, followed by the name of the polyatomic ion. For example, KNO3 is potassium nitrate, where nitrate is the polyatomic ion.