Atomic Structure Worksheet 2 Answer Key Revealed
Understanding the intricacies of atomic structure is fundamental for anyone studying chemistry. A key component of this understanding involves mastering the basic principles of atomic structure through worksheet exercises. Here, we dive deep into the second worksheet on atomic structure, providing not only the answers but also comprehensive explanations to help learners grasp the concepts thoroughly.
Part I: Atoms and Elements

In this section, we'll explore atoms, elements, and their fundamental properties.
Atomic Structure Basics

- What are atoms? Atoms are the smallest units of matter that maintain the chemical identity of an element.
- What are elements? Elements are substances consisting of atoms which all have the same number of protons.
💡 Note: Here are key terms worth remembering:
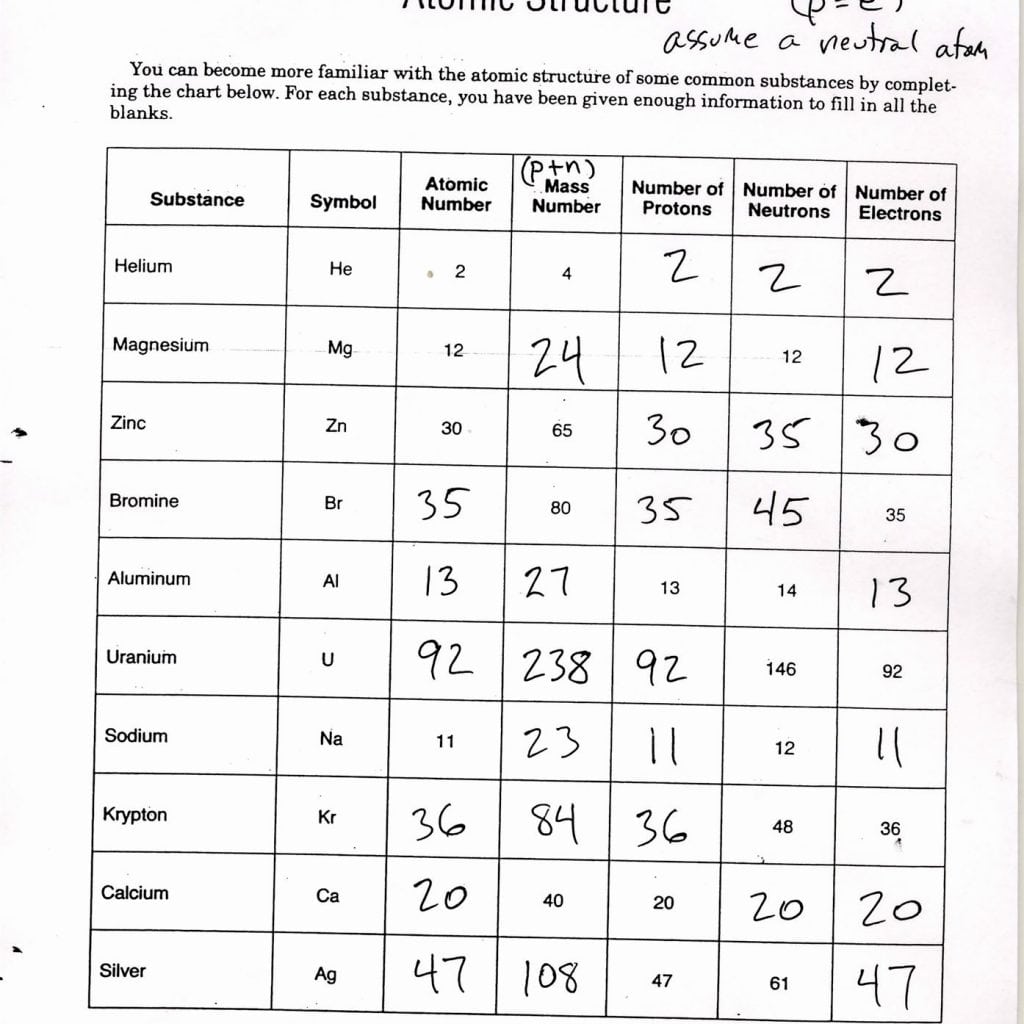
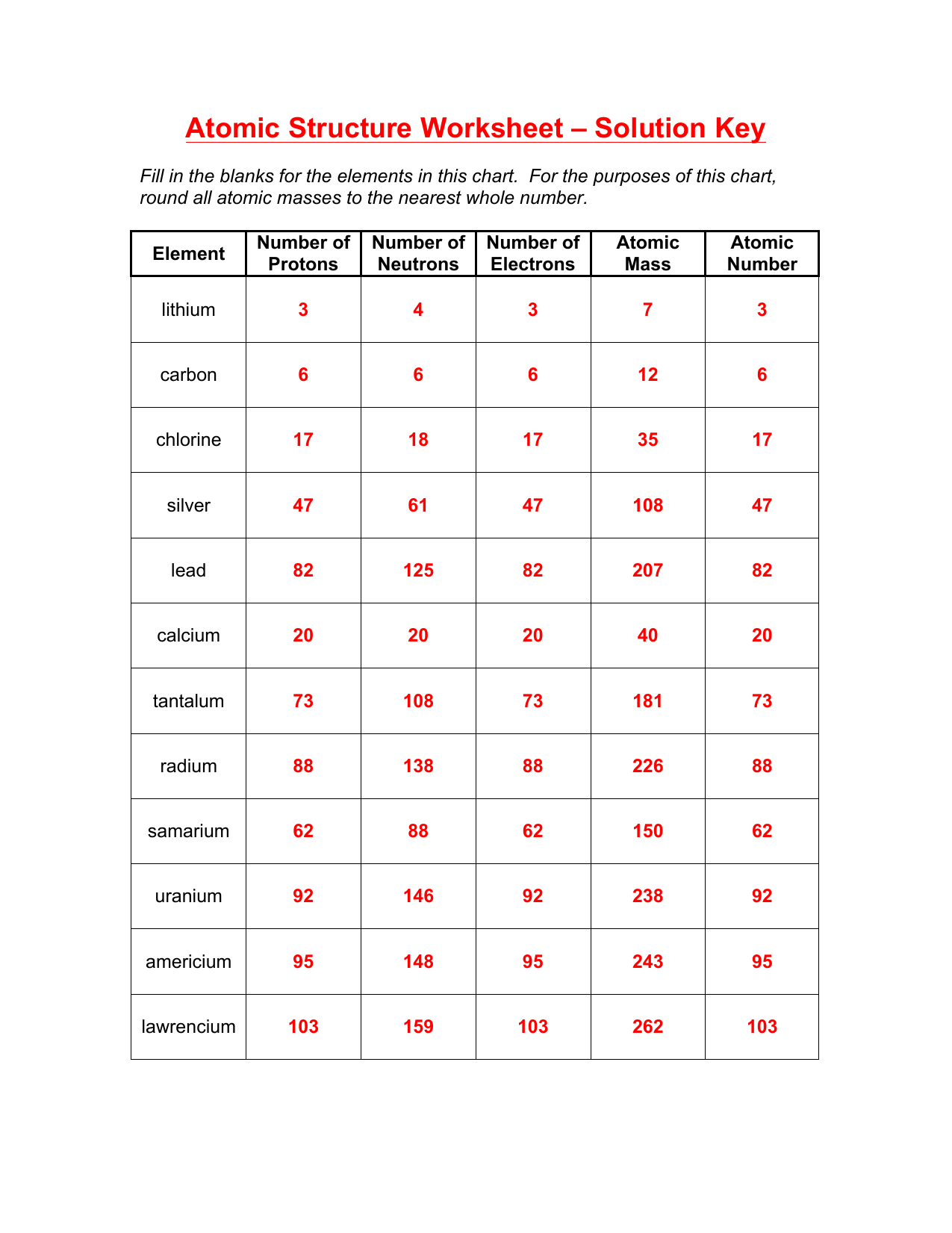
- Atomic number: The number of protons in an atom's nucleus.
- Mass number: The total number of protons and neutrons in an atom.
- Isotopes: Atoms of the same element with different numbers of neutrons.
Isotopes and Atomic Mass
An isotope's mass is determined by the sum of its protons and neutrons. Here's how to calculate atomic mass:
- Identify the mass numbers of all isotopes.
- Consider the relative abundance of each isotope.
- Apply the following formula:
Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + ...
💡 Note: Ensure you use the correct units (amu or u) when calculating atomic mass.
Part II: Understanding Electron Configuration

Electrons are arranged in shells around the nucleus of an atom. This configuration impacts how elements interact chemically.
Electron Shells

- The first shell can hold up to 2 electrons.
- The second shell can hold up to 8 electrons.
- Higher shells follow the rule of 2n² where n is the shell number.
Periodic Table Trends

| Property | Trend |
|---|---|
| Atomic Radius | Increases down a group, decreases across a period |
| Ionization Energy | Increases across a period, decreases down a group |
| Electronegativity | Increases across a period, decreases down a group |
Part III: Exploring Atomic Theory

Atomic theory has evolved over time, from the early ideas of Dalton to the modern quantum mechanical model.
Dalton's Atomic Theory

- All matter is composed of atoms.
- Atoms are indivisible and indestructible.
- All atoms of a given element are identical in mass and properties.
- Compounds are formed when atoms of different elements combine in simple, whole number ratios.
Modern Atomic Theory

With the advent of quantum mechanics, our understanding of atoms has become more complex and accurate:
- Wave-particle duality: Electrons exhibit both wave-like and particle-like properties.
- Probability clouds: Electrons have a probability of being found in certain regions around the nucleus.
- Quantum numbers: These define the electron's location and energy within the atom.
Wrapping Up

Understanding atomic structure requires continuous learning, practice, and application. Through this detailed exploration of atomic structure, we've provided answers and insights into the fundamental building blocks of chemistry. By mastering these concepts, you're better equipped to tackle more complex chemical phenomena and reactions.
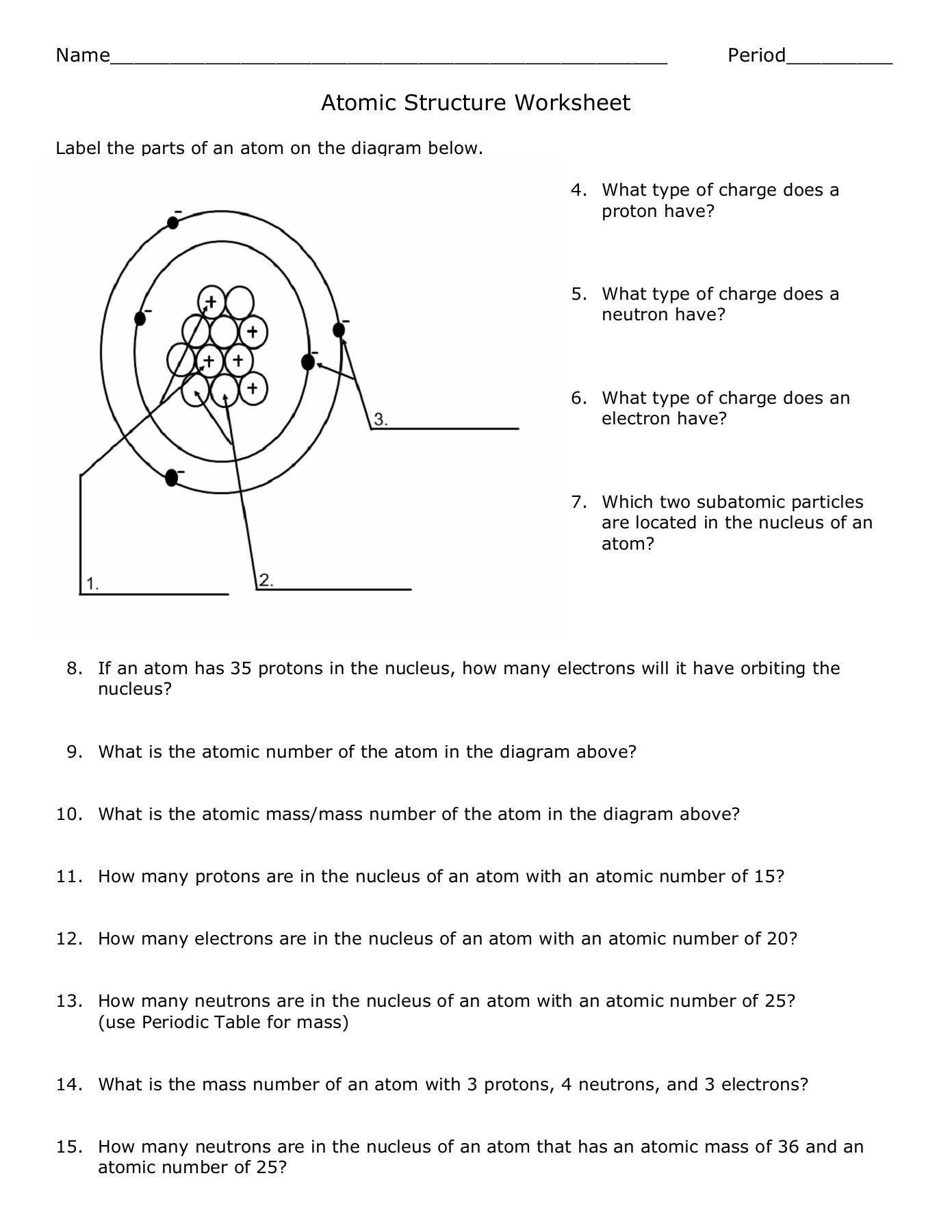
What is the significance of atomic number?

+
The atomic number uniquely identifies an element, as it represents the number of protons in the nucleus. This number determines the chemical behavior of an element.
How do isotopes affect atomic mass?

+
Isotopes have different mass numbers due to varying numbers of neutrons. Their relative abundances are used to calculate the average atomic mass of an element.
Why do atoms lose or gain electrons?

+
Atoms tend to gain or lose electrons to achieve a stable electron configuration, typically aiming for a full outer shell to increase stability.
What is the difference between atomic mass and atomic number?

+
The atomic number is the number of protons in an atom’s nucleus, while atomic mass is the average mass of all isotopes of that element considering their abundances.
Can you explain electron shells?
+Electron shells, or energy levels, describe where electrons orbit around the nucleus. Each shell has a specific maximum number of electrons it can hold.