5 Essential SI Unit Conversions for Students

Introduction to SI Units

The International System of Units, commonly known as the SI system, serves as the standard for measurement worldwide. This system simplifies scientific communication by ensuring that measurements are consistent and understandable across various fields. For students entering the realms of science, engineering, and technology, mastering SI units is not just beneficial but crucial. Let’s delve into five essential SI unit conversions that every student should know to excel in their academic and professional pursuits.
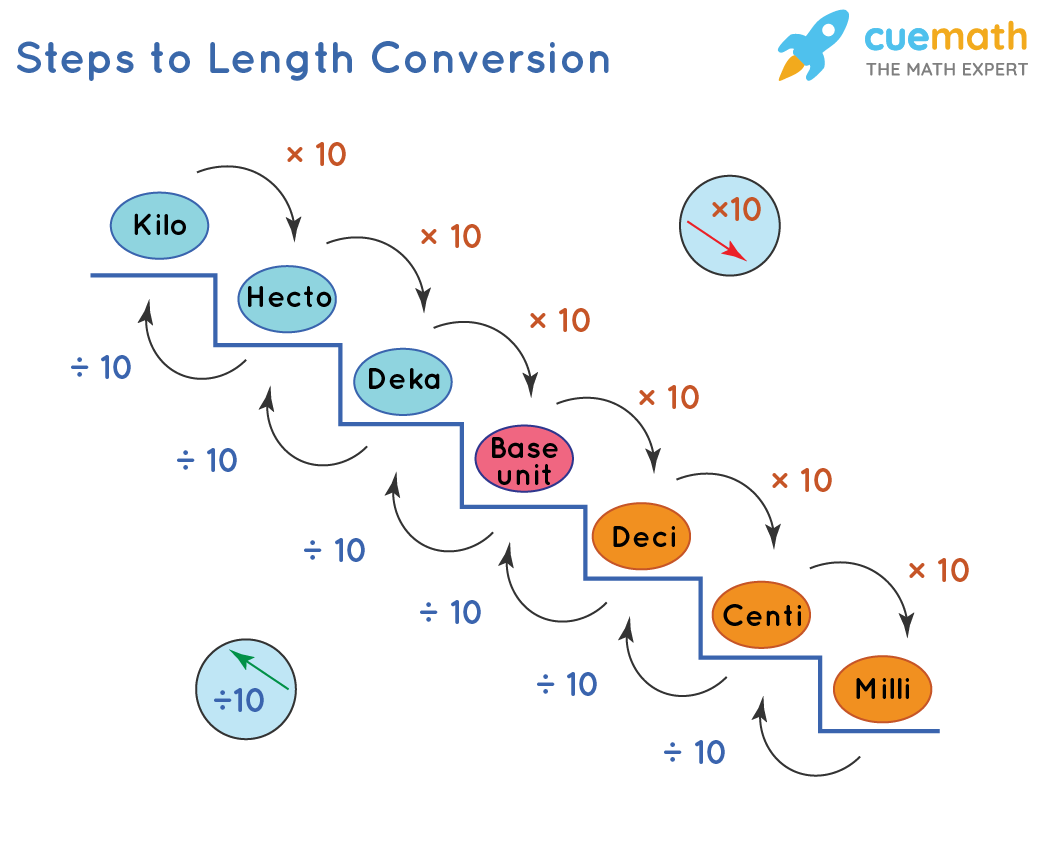
1. Length: Meters to Miles and Kilometers

Length measurement is fundamental in science and everyday life. Here’s how to convert between different units:
- Meter to Mile: 1 meter = 0.0006214 mile
- Meter to Kilometer: 1 meter = 0.001 kilometer
Understanding these conversions can help in real-world scenarios like interpreting distances in travel, construction, or even in running events:
- Travel distance: If you're planning to drive from Paris to Berlin, knowing the distance in miles or kilometers can make a difference in route planning.
- Construction: On a construction site, dimensions need to be precise, and converting from meters to feet or miles can be necessary.
✅ Note: For everyday conversions, especially in the USA, miles are more commonly used, whereas kilometers are the standard in most countries outside of the USA.
2. Mass: Grams to Pounds and Ounces

Mass, or the measure of how much matter an object contains, is a critical quantity in physics and chemistry:
- Gram to Pound: 1 gram ≈ 0.0022046 pound
- Gram to Ounce: 1 gram ≈ 0.035274 ounce
Conversions in mass are crucial when dealing with substances:
- Chemistry: When measuring chemicals, understanding weight conversions is key for accurate experiments.
- Cooking: Recipes often list ingredients in grams, but some kitchen scales might display weights in ounces or pounds.
3. Volume: Liters to Gallons and Cubic Feet

Volume measurements are important in disciplines from health sciences to engineering:
- Liter to Gallon (US): 1 liter ≈ 0.264172 gallon
- Liter to Cubic Foot: 1 liter ≈ 0.0353147 cubic foot
Practical applications include:
- Fluid Medicine Dosage: Understanding liter to gallon conversion can help in administering correct dosages.
- Transportation: Determining fuel capacity or cargo space often involves converting volume units.
4. Temperature: Kelvin to Celsius and Fahrenheit

Temperature measurements are essential in scientific research, food preparation, and climate science:
- Kelvin to Celsius: °C = K - 273.15
- Kelvin to Fahrenheit: °F = (K - 273.15) × 9/5 + 32
Applications include:
- Scientific Experiments: Converting between Kelvin and Celsius is common in thermodynamics.
- Global Weather Reports: Understanding temperature scales allows for comprehension of weather patterns across different countries.
🌡️ Note: Always use the absolute zero (-273.15°C or 0 K) as a reference point for temperature conversions to Kelvin.
5. Energy: Joules to Calories and Watt-hours

Energy is a fundamental concept in physics:
- Joule to Calorie: 1 joule ≈ 0.239 calories
- Joule to Watt-hour: 1 joule = 0.000277778 watt-hour
These conversions are useful in:
- Health and Nutrition: Understanding calorie content in food or energy expenditure in exercise.
- Power Consumption: Calculating energy usage in electrical devices like smartphones or laptops.
In our journey through these essential SI unit conversions, students gain the tools to navigate through various academic disciplines and real-life situations.
As we’ve explored, understanding these conversions allows for:
- Improved academic performance in science and engineering courses.
- Better grasp of everyday tasks involving measurements.
- Seamless communication with professionals in international settings.
Mastery of SI unit conversions is not merely about numbers but about gaining a comprehensive understanding of the measurements that frame our world. This knowledge equips students to solve problems, make precise calculations, and contribute effectively to scientific endeavors. By familiarizing themselves with these conversions, students are better prepared to tackle the complexities of modern science and technology.
In wrapping up this comprehensive look at essential SI unit conversions, it’s evident that these skills are not just academic requirements but essential tools for life.
Why do we use the SI system?

+
The SI system ensures consistency in measurements, making scientific and technical communication clear and universally understandable. It promotes precision and reduces the chances of misunderstanding or errors due to differing measurement standards.
How can I remember SI unit conversions easily?

+
Practice regularly, use mnemonic devices, and understand the context or real-life applications of the conversions. Relating the units to everyday scenarios can also enhance retention.
Is there an app or tool for SI unit conversions?
+
Yes, there are numerous apps and online tools available like Google Converter, Unit Converter apps, and websites like Omni Calculator. These tools can instantly convert units, helping students to understand the relationships between them.