5 Ways to Master Predicting Chemical Reactions

Understanding chemical reactions is fundamental for students of chemistry, scientists, and even for industrial applications. Predicting how chemicals will react with each other is not just about mixing substances and observing changes; it's an art backed by principles of thermodynamics, kinetics, and quantum mechanics. In this comprehensive guide, we'll explore five effective strategies to enhance your ability to predict chemical reactions accurately. This journey will not only solidify your understanding of chemistry but also make the prediction process a bit more intuitive and less daunting.
1. Learn the Basics of Chemical Reactivity

Mastering chemical reactions starts with a solid foundation in basic chemical principles:
- Periodic Table Knowledge: Understand the electronic configurations, electronegativities, and reactivity trends of elements. Elements at different ends of the periodic table exhibit vastly different behaviors.
- Types of Chemical Bonds: Ionic, covalent, metallic, and hydrogen bonds; knowing how atoms bond together is crucial for predicting reaction behavior.
- Polarity and Solubility: Polar substances are more likely to dissolve in polar solvents, a concept essential for understanding reaction environments.
🔬 Note: Always consider the state (solid, liquid, gas) of reactants since it can significantly affect reaction outcomes.
2. Master Reaction Mechanisms

A reaction mechanism outlines the step-by-step process through which reactants are transformed into products:
- Substitution Reactions: Common in organic chemistry, where one functional group is substituted by another.
- Elimination Reactions: Characterized by the removal of small molecules like water or hydrogen halide from organic compounds.
- Addition Reactions: Typically involving the addition of a molecule across a double or triple bond.
- Redox Reactions: Where electron transfer occurs between species, often involving changes in oxidation states.
To become proficient, you should:
- Study reaction intermediates and transition states.
- Memorize common arrow-pushing patterns for electron movement.
🎓 Note: Understanding the role of catalysts in speeding up reactions without being consumed can change the kinetics of the reaction significantly.
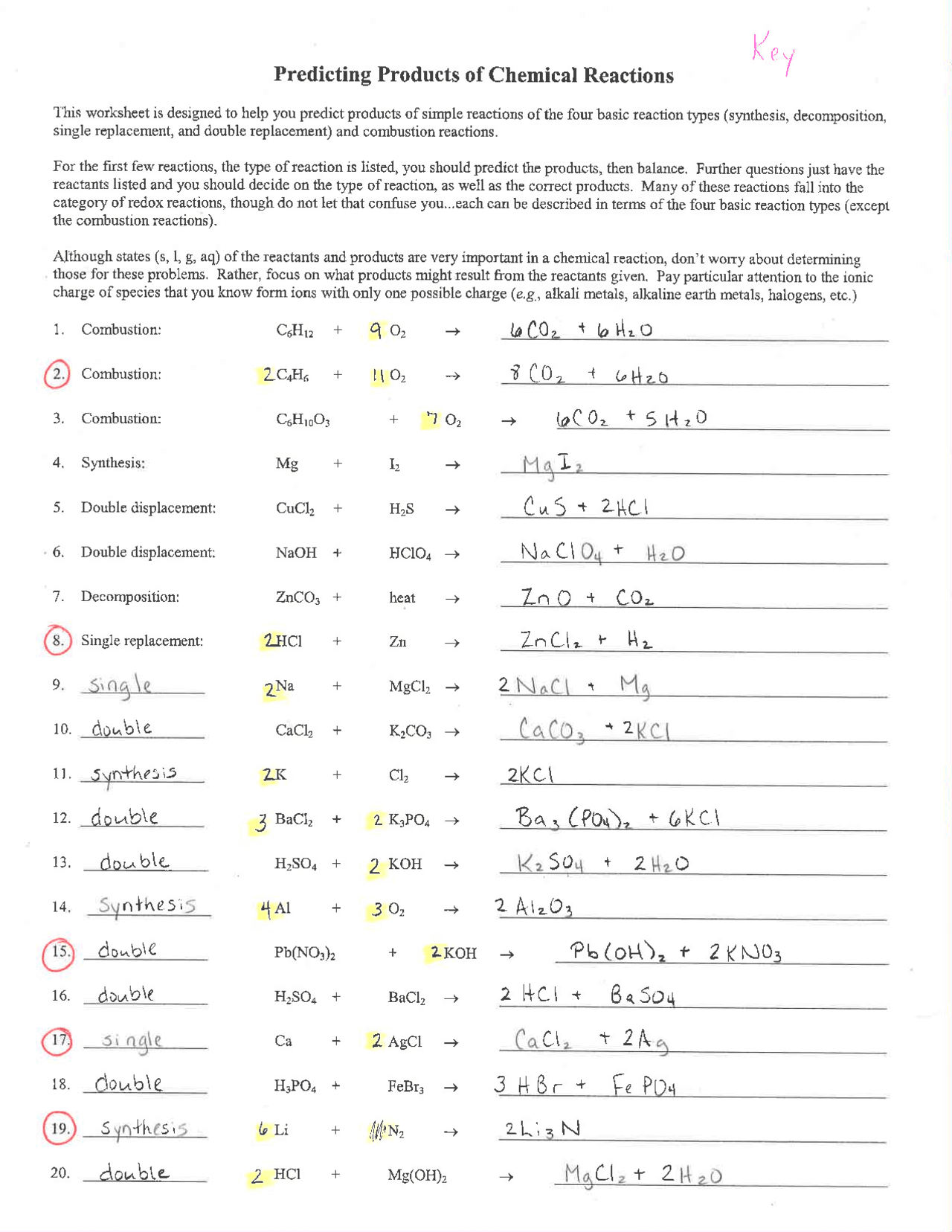
3. Utilize Predictive Tools and Models
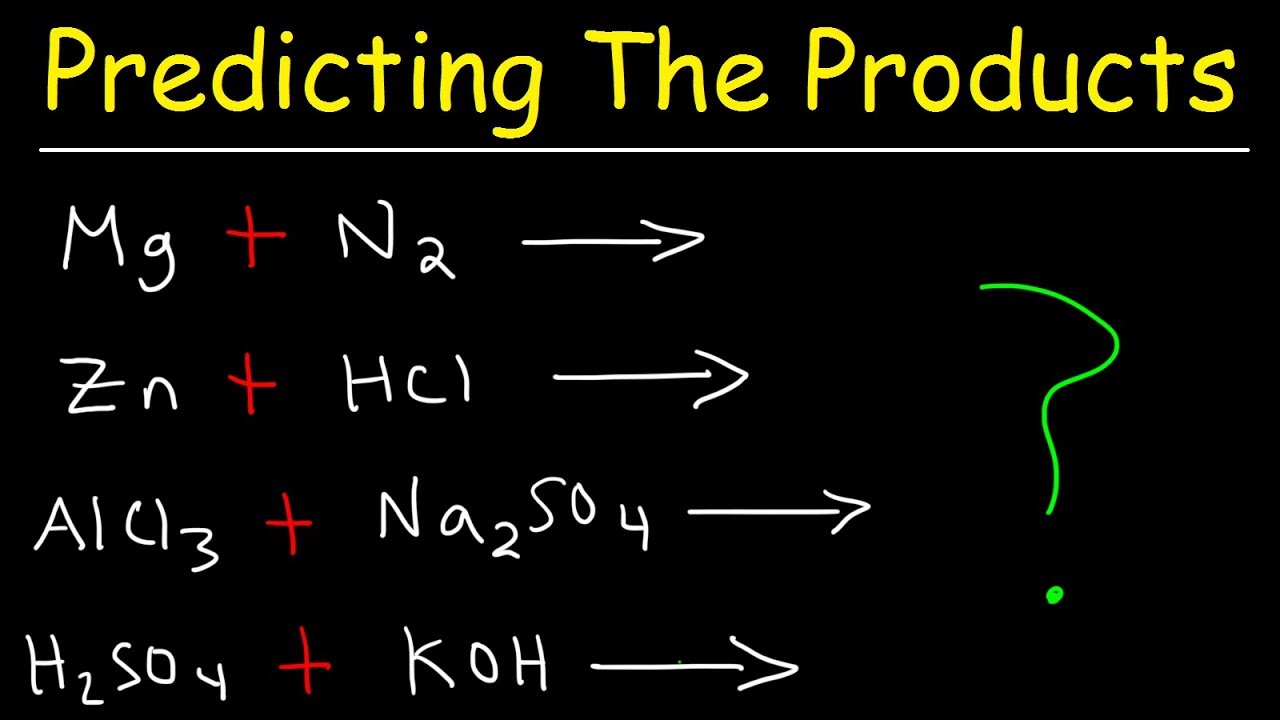
In the modern era, predicting chemical reactions isn’t limited to theoretical knowledge alone:
- Chemical Modeling Software: Tools like Gaussian or ChemDraw can help visualize molecular structures and simulate reactions.
- Online Calculators: Websites offering reaction prediction calculators based on algorithms or machine learning models can provide quick insights.
- Quantum Mechanical Calculations: For more complex predictions, quantum chemistry software like Q-Chem or GAMESS can be employed.
These tools not only enhance your predictive accuracy but also provide visual aids to understand the mechanisms at play.
4. Practice with Real-World Scenarios

Real-world scenarios offer an invaluable learning platform:
- Laboratory Work: Performing experiments firsthand allows you to observe how reactions actually proceed.
- Industrial Applications: Understanding how reactions are utilized in industry can give a practical perspective on chemical reactions.
- Case Studies: Analyze documented cases of reactions for both successes and failures to learn from real-life examples.
While tools and models provide theoretical understanding, nothing beats the practical insight gained through direct observation or working with case studies.
5. Reflect and Iterate

The final and perhaps the most iterative step involves:
- Revisiting: Go back to basics, tools, mechanisms, or scenarios where you’ve had difficulties.
- Reevaluation: Assess what went wrong or right in your predictions, focusing on what might be improved.
- Peer Review: Engage with a community or peers to discuss and learn from others’ experiences in predicting reactions.
This reflective practice not only polishes your predictive skills but also keeps you informed about the ever-evolving field of chemistry.
Through these five approaches, you're setting yourself up to become adept at predicting chemical reactions. From learning the fundamental principles to employing advanced tools and reflecting on your learning journey, you'll find that with each step, your intuition for chemical behavior sharpens. Remember, chemistry is not just about equations and memorization; it's about understanding the language of matter and how it interacts.
Mastering the art of predicting chemical reactions has numerous benefits. It can lead to safer experimentation, better design of chemical processes, and even innovations in fields like drug discovery or environmental science. With these tools and approaches in your arsenal, you're well on your way to unlocking the secrets of chemical reactions, making them less of a mystery and more of a well-understood symphony of atoms and molecules.
How important is understanding chemical reaction mechanisms?
+
Understanding reaction mechanisms is crucial as they provide insight into the how and why of chemical transformations, allowing for better prediction and control over reactions.
Can I predict chemical reactions without software tools?

+
Yes, with a solid understanding of chemical principles, you can make predictions, but tools can enhance accuracy and provide a deeper understanding.
What are some common pitfalls in predicting chemical reactions?

+
Some common pitfalls include: overlooking secondary reactions, not accounting for reaction conditions, neglecting solvent effects, and underestimating kinetic factors.
How do real-world scenarios improve prediction skills?

+
Real-world scenarios provide practical insights into how reactions behave under different conditions, offering experience that theoretical knowledge alone cannot provide.
Why is it important to iterate and reflect on your predictions?

+
Iteration and reflection allow you to learn from your mistakes, improve your understanding, and develop a more nuanced approach to predicting chemical reactions.