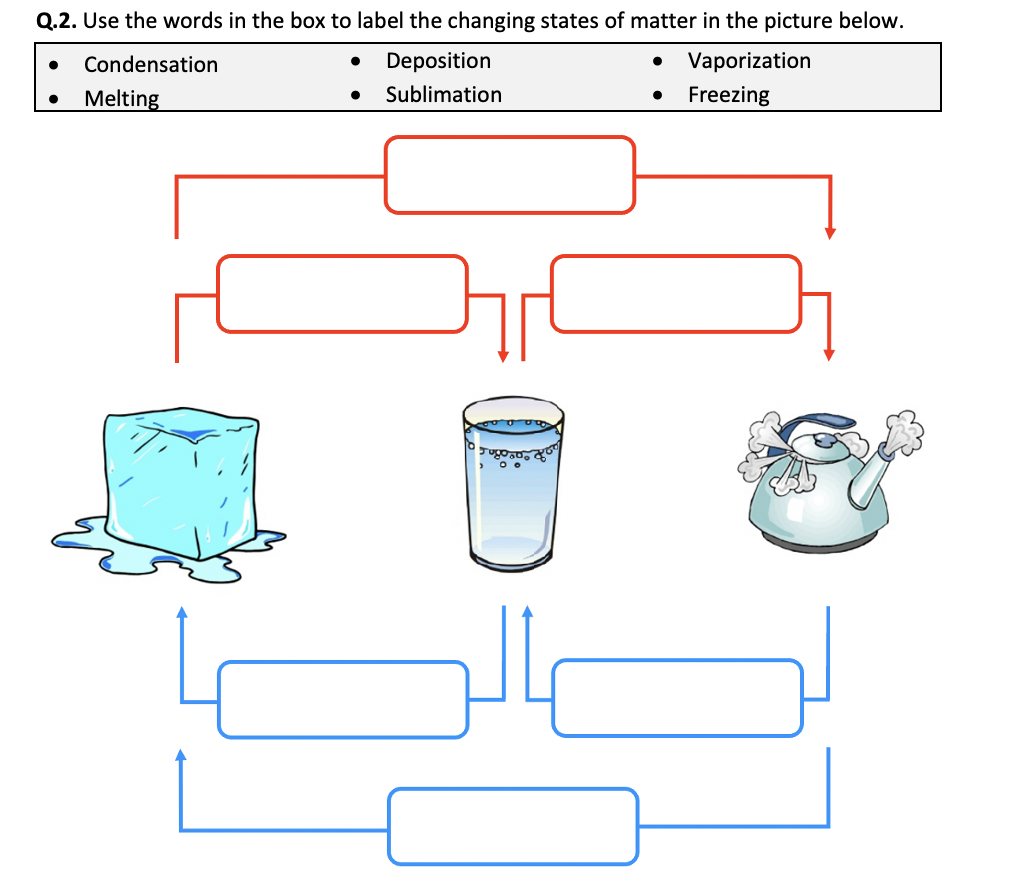
5 Key Insights from Phase Change Graph Worksheets

Exploring the phase change or phase transition of substances is fundamental in both academic and applied science, offering insights into how materials respond to changes in temperature, pressure, or other environmental factors. Phase change graph worksheets serve as a critical educational tool to visualize these transitions and understand their implications. Here, we delve into five key insights that can be gleaned from studying these worksheets, enriching your understanding of how substances like water or metals alter their state and the practical applications this knowledge has.
Insight 1: Understanding Phase Change Concepts

Phase change graphs, often called heating curve or cooling curve diagrams, illustrate how a substance’s temperature changes as heat is added or removed. These graphs typically show several distinct phases:
- Solid phase: Where the substance is in its lowest energy state, often as a solid.
- Melting (Fusion): The transition from solid to liquid, where the temperature stays constant until the phase change is complete.
- Liquid phase: The substance exists as a liquid, with temperature increasing with heat.
- Boiling (Vaporization): Here, the transition from liquid to gas occurs, again at a constant temperature.
- Gas phase: Where the substance is in its gaseous form, with temperature continuing to rise.
The latent heat or energy required to change phases without altering temperature is crucial. Phase change graphs help students visualize and understand these concepts, emphasizing how energy is absorbed or released during these transitions.
💡 Note: Each phase transition has a specific name and associated terminology, which are important to remember for a clear understanding of the process.
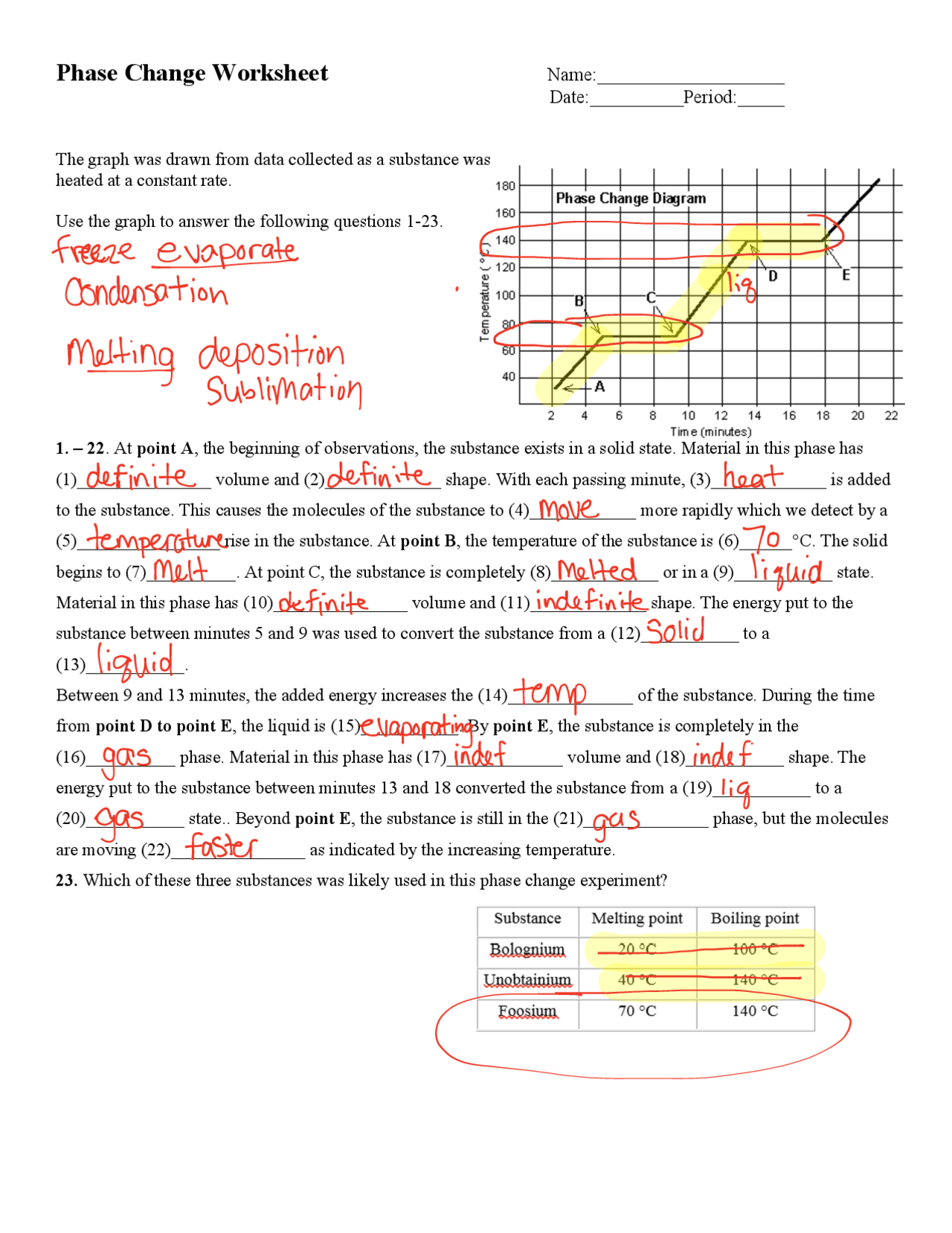
Insight 2: Identifying Critical Temperatures

Key phase transition temperatures like the melting point and boiling point are immediately visible on a phase change graph:
| Phase Change | Terminology |
|---|---|
| From Solid to Liquid | Melting Point (Fusion) |
| From Liquid to Gas | Boiling Point (Vaporization) |
| From Liquid to Solid | Freezing Point |
| From Gas to Liquid | Condensation Point |

These points are critical because they define the conditions under which a substance changes phase, allowing scientists and engineers to predict behavior under various conditions.
Insight 3: Energy Considerations
The phase change graph is invaluable for understanding the energy dynamics involved in phase transitions:
- The latent heat of fusion is the energy needed to melt a solid into a liquid without a change in temperature.
- The latent heat of vaporization represents the energy to turn a liquid into a gas at a constant temperature.
This insight is essential for practical applications like controlling energy inputs in industrial processes or designing cooling systems that depend on the cooling effect of latent heat release during condensation.
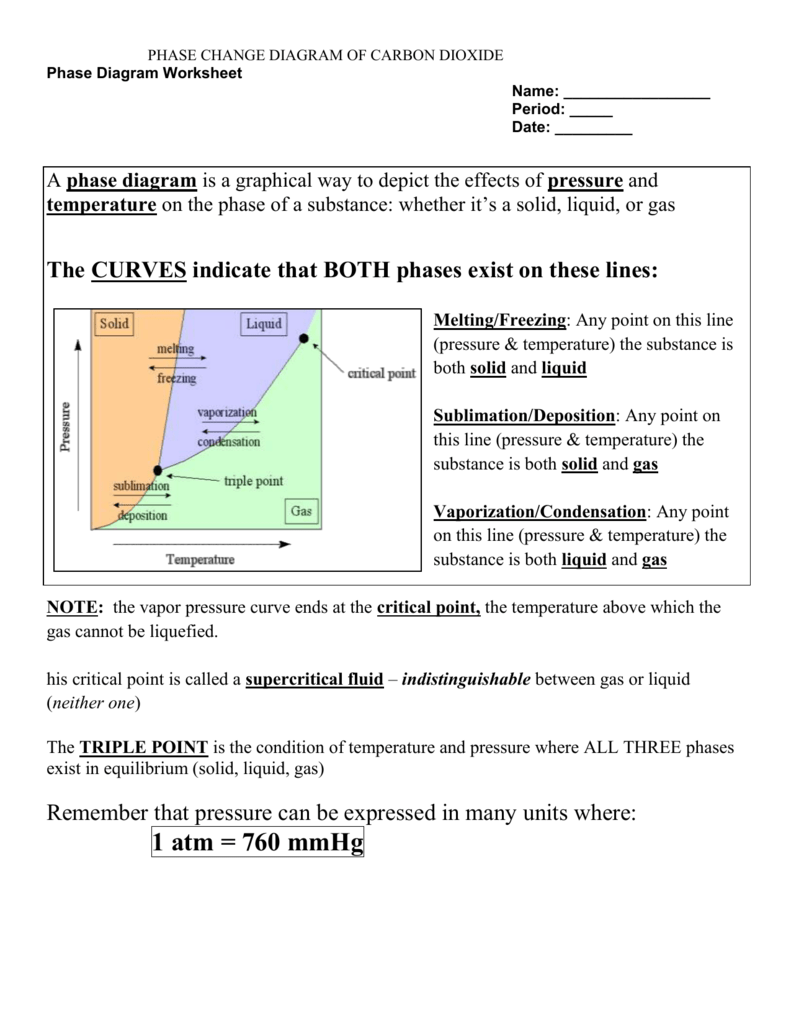
Insight 4: Predicting Physical Properties
Phase change graphs provide a visual representation that helps predict physical properties:
- The duration of phase changes can be predicted, which is vital for processes like cooking, heating, and cooling.
- Understanding how substances behave at different temperatures helps in selecting materials for applications like thermal insulation or in heat exchangers.
By studying these graphs, one can estimate the energy requirements for heating or cooling processes, which is crucial in engineering design and energy management.
🔍 Note: Different substances will have unique phase transition behaviors, which are important to consider when designing systems or materials.
Insight 5: Real-World Applications

The insights from phase change graphs are not confined to the lab; they have real-world applications:
- Refrigeration and Air Conditioning: Systems exploit the phase change properties of refrigerants to cool environments by absorbing heat when they evaporate and releasing it when they condense.
- Heat Recovery Systems: Engineers use phase change materials (PCMs) to store and release thermal energy, reducing energy costs.
- Food Processing: Understanding phase changes helps in controlling cooking temperatures, freezing, and thawing food items safely.
Phase change graphs are more than educational tools; they are foundational in our understanding of how the physical world works and how we can manipulate it for our benefit. Through careful study and analysis of these graphs, students, researchers, and engineers can glean profound insights into the behavior of materials, enabling them to design systems and processes that are not only efficient but also innovative. The dynamic interaction between energy and matter, as visualized in these graphs, is at the heart of many scientific and technological advancements, from basic chemistry lessons to complex industrial applications.
Why are phase change graphs important in education?

+
Phase change graphs visually represent how substances change state, helping students understand the energy requirements for these transitions, and the behavior of materials at different temperatures.
Can phase change graphs be applied in real-world scenarios?

+
Absolutely! Understanding phase transitions is essential for processes like cooking, refrigeration, HVAC system design, and thermal energy storage using phase change materials.
How do latent heat and phase change relate?
+
Latent heat is the energy required to change the phase of a substance without changing its temperature. Phase change graphs show this as horizontal lines during phase transitions, illustrating the energy absorbed or released.



