5 Tips for Naming Hydrocarbons Easily
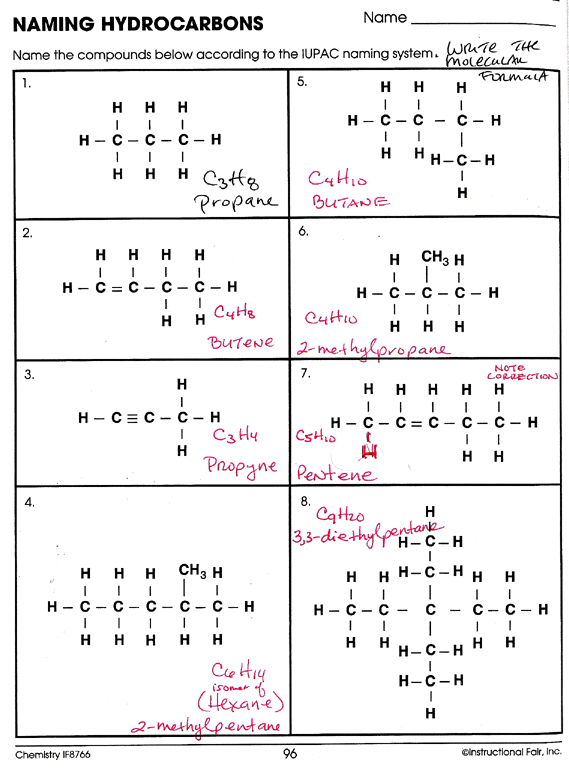
The naming of hydrocarbons is an essential skill for students and professionals in chemistry. Mastering this skill can make understanding and communicating complex chemical structures much more straightforward. Here are five tips to help you name hydrocarbons with ease:
Ethylene Glycol instead of. Master the Basics

Before delving into the complex world of hydrocarbon naming, it's crucial to have a solid grasp on the basics:
- Learn the prefixes: Know the prefixes for different numbers of carbon atoms (meth-, eth-, prop-, but-, pent-, and so on).
- Identify functional groups: Be familiar with common groups like alcohols (OH), carboxylic acids (COOH), amines (NH₂), etc.
- Memorize the core rules: Understand IUPAC (International Union of Pure and Applied Chemistry) rules which govern the naming of organic compounds.
⚗️ Note: Naming hydrocarbons involves both the identification of structural features and adherence to IUPAC conventions.
2. Break Down the Structure

When faced with a hydrocarbon, break it down into manageable parts:
- Count the total number of carbon atoms to determine the parent chain.
- Identify the longest continuous chain of carbon atoms as the parent alkane.
- Locate any functional groups or substituents on the chain.
Organize your observations systematically. This approach simplifies what might initially seem like a complex structure into something much more manageable.

3. Use Systematic Naming

The IUPAC system is systematic and logical, designed for consistency:
- Number the carbons: Start numbering from the end that gives the lowest numbers to substituents.
- Identify and list substituents: These are alkyl groups or other atoms/groups replacing hydrogen atoms on the parent chain.
- Use numerical prefixes: If there are multiple substituents of the same type, use di-, tri-, etc.
- Determine the suffix: Based on the highest priority functional group (-ane for alkanes, -ene for alkenes, -yne for alkynes, etc.)
Example:
| Structure | Naming |
|---|---|
| CH₃CH₂CH(CH₃)CH₂CH₃ | 2-Methylpentane |
| CH₂=CHCH₂CH₂CH₃ | 1-Pentene |
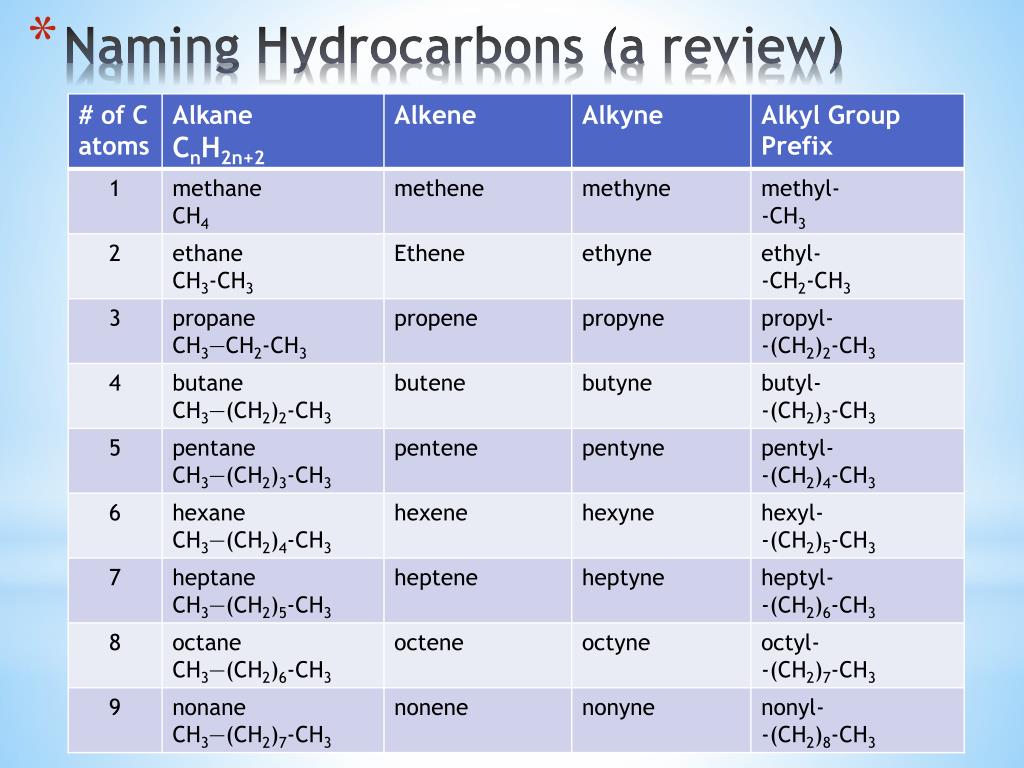
💡 Note: The IUPAC system provides a clear framework for naming hydrocarbons, making it easier to communicate and understand molecular structures.
4. Practice with Examples
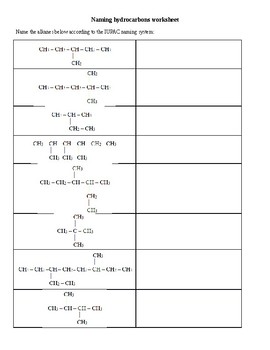
Like any language, practice enhances fluency. Here are some examples:
- Alkanes: CH₃CH₂CH₃ (Propane), CH₃CH₂CH₂CH₃ (Butane)
- Alkenes: CH₂=CH₂ (Ethene), CH₃CH=CH₂ (Propene)
- Alkynes: CH≡CH (Ethyne), CH₃C≡CH (Propyne)
- Cyclic compounds: Cyclohexane, Cyclopentene
Regularly practice drawing and naming structures from both simple to more complex hydrocarbons to improve your confidence and accuracy.
5. Leverage Mnemonic Devices

To make naming easier, use mnemonics:
- Prefixes: Mnemonic - “Moms Enjoy Playing Bingo” (Meth-, Eth-, Prop-, But-, Pent-).
- Functional Groups: A memorable phrase like “Alcohols Can Always Laugh” (Alcohols, Carboxylic acids, Aldehydes, Ketones).
- Alkene/Eyne: “Ethyl has ends, Ethyne has two ends” to remember the numbering for placement.
🎨 Note: Mnemonics can be personalized to your learning style, making complex names easier to remember.
By mastering these techniques, naming hydrocarbons becomes not only more manageable but also a fascinating journey into the language of chemistry. Through consistent practice and application of these tips, you'll be able to efficiently name any hydrocarbon you encounter, enhancing your understanding and communication of organic chemistry concepts.
What are the common suffixes used in hydrocarbon naming?

+
The suffixes used in hydrocarbon naming include -ane for alkanes, -ene for alkenes (indicating a double bond), and -yne for alkynes (indicating a triple bond). For example, methane, ethene, and propyne.
How do you determine the parent chain?
+
The parent chain is the longest continuous chain of carbon atoms in a molecule. Numbering should start from the end closest to a substituent or functional group to give the lowest possible numbers to these groups.
What is the importance of naming hydrocarbons systematically?

+
Systematic naming ensures clear communication among chemists worldwide. It helps in avoiding ambiguity and accurately describes the molecule’s structure, functionality, and properties, which is vital in chemical research and industry.