5 Simple Tricks for Naming Acids Correctly

Naming acids correctly is a fundamental skill for chemistry students, lab technicians, and anyone involved in chemical sciences. The task, though it might seem daunting at first, can be simplified through the understanding of some basic rules. Let's dive into five simple tricks to master the art of naming acids, which will not only improve your chemical nomenclature but also streamline your communication with fellow chemists.
Understanding Acid Types

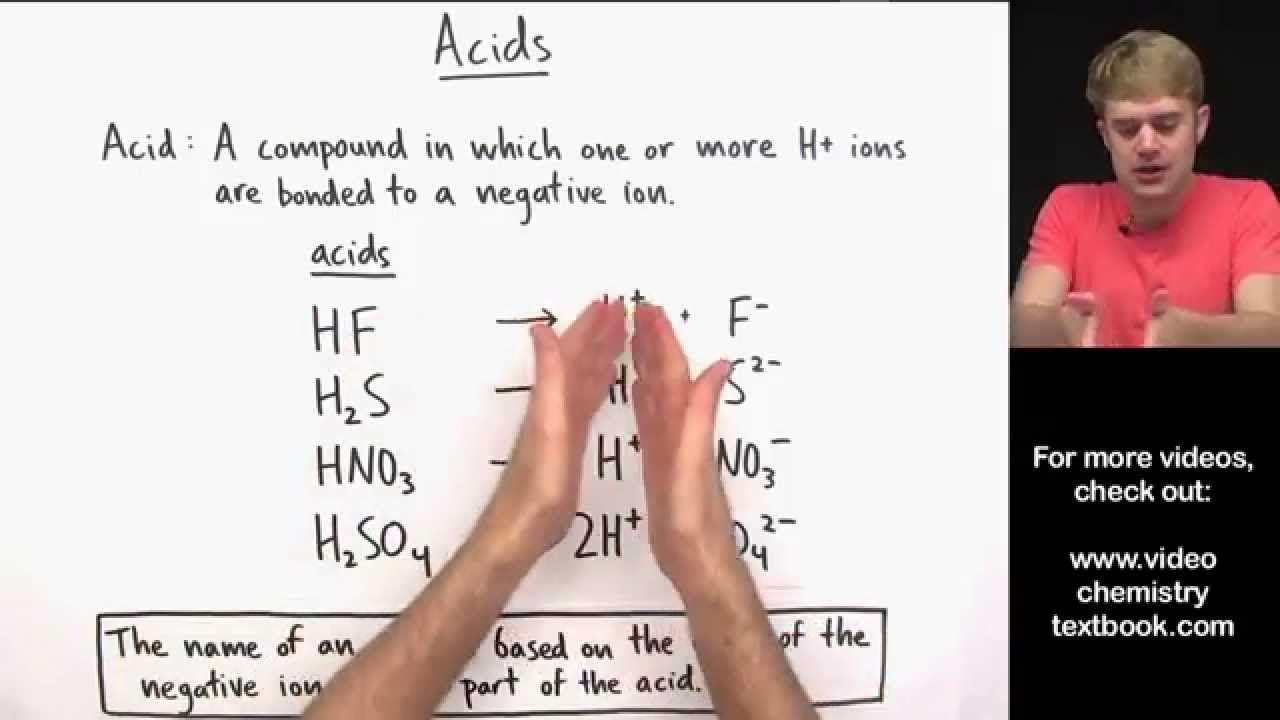
The first step to naming an acid correctly is identifying its type:
- Binary Acids: These are acids composed of hydrogen and a non-metal.
- Oxyacids: Also known as ternary acids, these include hydrogen, oxygen, and another element, usually a non-metal or a polyatomic ion.
Binary Acids

Binary acids consist of hydrogen and one non-metal atom. Here’s how to name them:
- Start with “Hydro”: All binary acids begin with the prefix “hydro-”.
- Add the Non-Metal: Follow with the stem of the non-metal’s name. For example, chlorine becomes “chlor”.
- End in “-ic Acid”: Conclude with “-ic acid” for the acid name.
⚛ Note: Remember, binary acids do not include oxygen.
Oxyacids

These acids contain hydrogen, oxygen, and a central atom. Here’s the breakdown for naming:
| Polyatomic Ion | Acid Ending |
|---|---|
| -ate (e.g., nitrate) | -ic acid (e.g., nitric acid) |
| -ite (e.g., nitrite) | -ous acid (e.g., nitrous acid) |
| per- prefix (e.g., perchlorate) | per-…-ic acid (e.g., perchloric acid) |
| hypo- prefix (e.g., hypochlorite) | hypo-…-ous acid (e.g., hypochlorous acid) |
Using Prefixes and Suffixes

In some cases, acids have prefixes and suffixes that help in their correct naming:
- The prefix per- indicates one more oxygen atom than usual.
- The prefix hypo- indicates one less oxygen atom than usual.
- -ate becomes -ic when transitioning to an acid.
- -ite becomes -ous when transitioning to an acid.
Recognize Common Exceptions
Here are some common exceptions you might encounter:
- Hydrocyanic Acid (HCN): Although it’s a binary acid, it’s named differently due to its unique properties.
- Carbonic Acid (H2CO3): Despite having oxygen, it’s named after the central carbon atom rather than following the typical rules for ternary acids.
⚛ Note: Familiarity with these exceptions will prevent errors in naming acids.
Practice with Real-Life Examples

Understanding the rules is one thing, but applying them to real-life scenarios is what solidifies your knowledge. Here are some examples:
- HCl becomes hydrochloric acid.
- HNO3 (from nitrate ion) becomes nitric acid.
- H2SO4 (from sulfate ion) becomes sulfuric acid.
To summarize, these five simple tricks provide a structured approach to naming acids:
- Identify whether it's a binary or oxyacid.
- Use the appropriate prefixes and suffixes for oxyacids.
- Know when and how to use "hydro-" for binary acids.
- Remember to account for common exceptions.
- Practice with real-life chemical scenarios to reinforce learning.
By understanding these principles, you'll find that naming acids becomes more intuitive and less of a guessing game. Mastery of this aspect of chemistry not only aids in your own understanding but also ensures clear and unambiguous communication within the scientific community.
What is the difference between an -ate and an -ite acid?

+
The main difference is in the number of oxygen atoms. An -ate ion has more oxygen atoms than an -ite ion. When these ions become acids, -ate turns into -ic acid, and -ite turns into -ous acid.
How do you identify if an acid is binary or oxyacid?

+
Binary acids have only hydrogen and one non-metal. Oxyacids, or ternary acids, contain hydrogen, oxygen, and another element or a polyatomic ion.
What does the prefix “hydro-” indicate?

+
The prefix “hydro-” is used to denote binary acids, which contain hydrogen and a non-metal with no oxygen atoms.
Are there any acids that don’t follow the standard naming rules?

+
Yes, some acids like hydrochloric acid (HCl) follow the binary acid rules but are known by their traditional names. For example, HCN is called hydrocyanic acid instead of hydrogen cyanide.