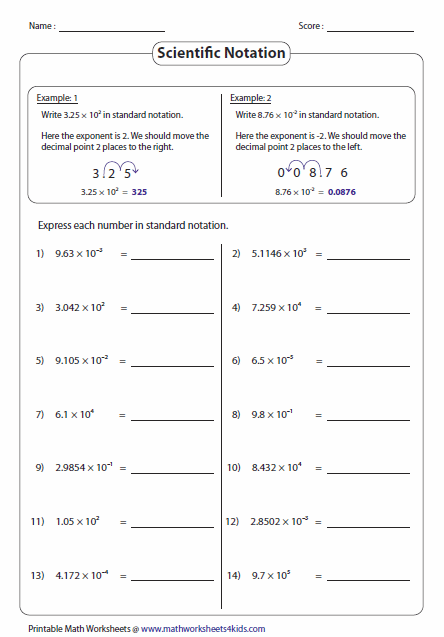
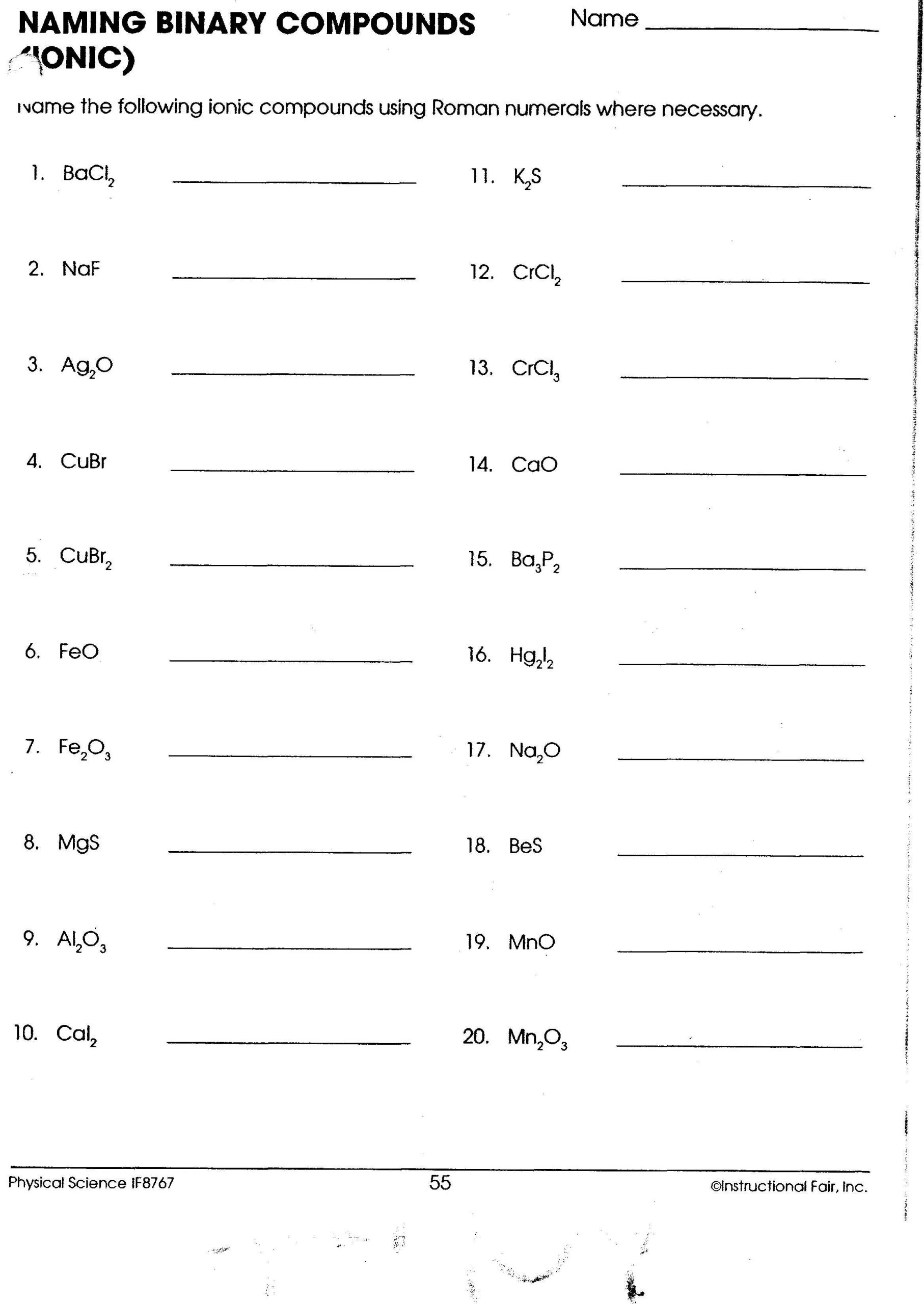
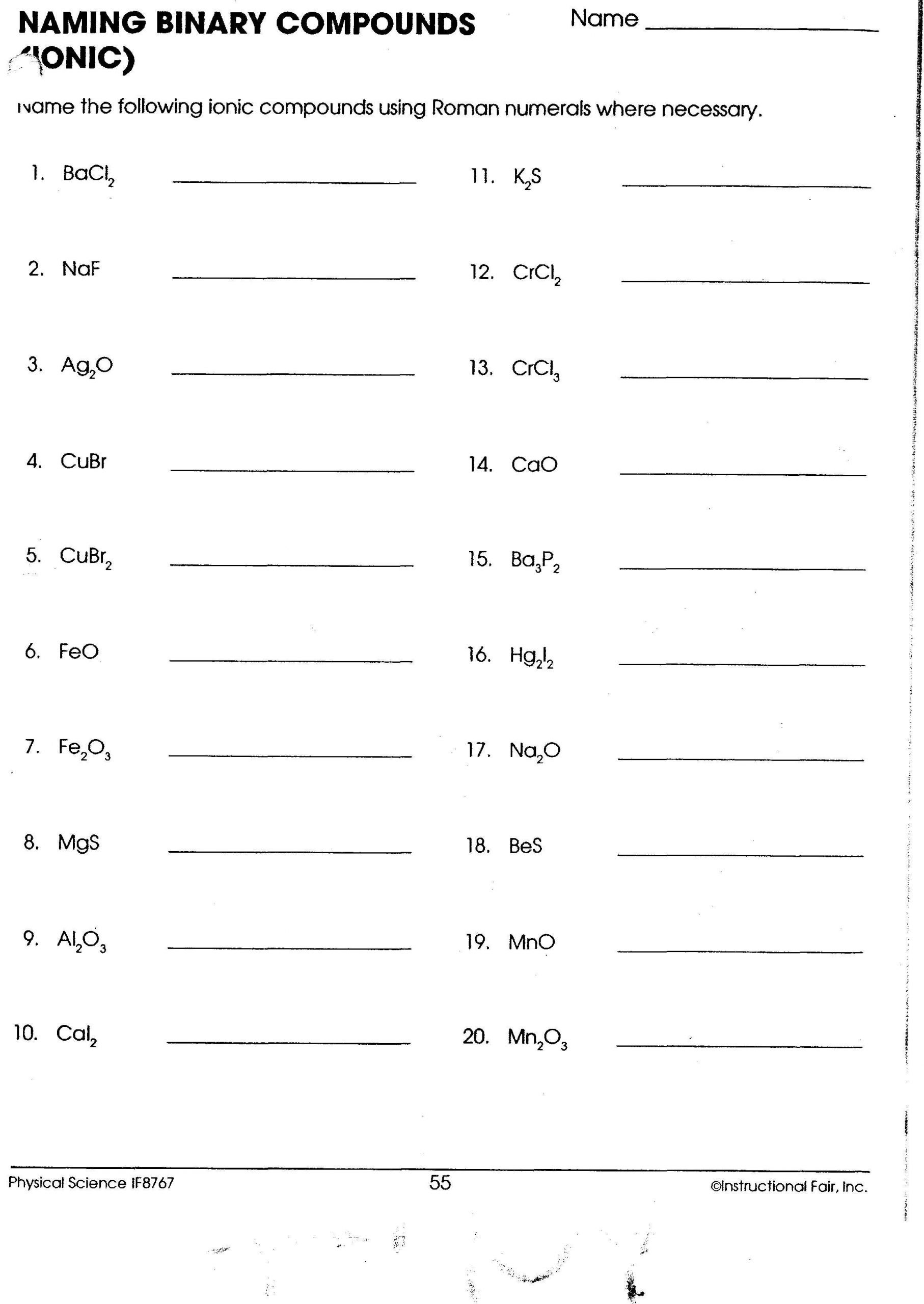
Mastering Ionic Compound Names: Essential Worksheet Guide

In the fascinating world of chemistry, mastering the art of naming compounds, particularly ionic compounds, is crucial. Whether you're a student learning basic chemistry or an enthusiast expanding your knowledge, understanding ionic compound names opens up the complexities of chemical reactions and bonding. This guide provides a comprehensive walkthrough on how to excel in your ionic compound worksheet with practical examples, easy steps, and key notes to remember.
Understanding Ionic Compounds

Before diving into the intricacies of naming, it's essential to understand what ionic compounds are:
- Ionic compounds are formed when metals lose electrons to non-metals which gain them, creating positive and negative ions, respectively.
- The electrostatic attraction between these ions forms the ionic bond.
- Common examples include sodium chloride (NaCl) and calcium fluoride (CaF2).
Basic Rules for Naming Ionic Compounds
Naming ionic compounds follows a systematic approach:
Monatomic Ions

- Cation: Name the metal ion as it appears on the periodic table. For transition metals, use Roman numerals to indicate the charge, if necessary (e.g., Iron(II) for Fe2+).
- Anion: For non-metals, change the ending of the element name to -ide. For example, chlorine becomes chloride (Cl-).
Polyatomic Ions

These ions consist of more than one atom. They retain their group name:
- Examples include nitrate (NO3-), sulfate (SO42-), and phosphate (PO43-).
- When naming, treat the polyatomic ion as a single unit, not altering its name.
Putting It Into Practice: A Worksheet Example
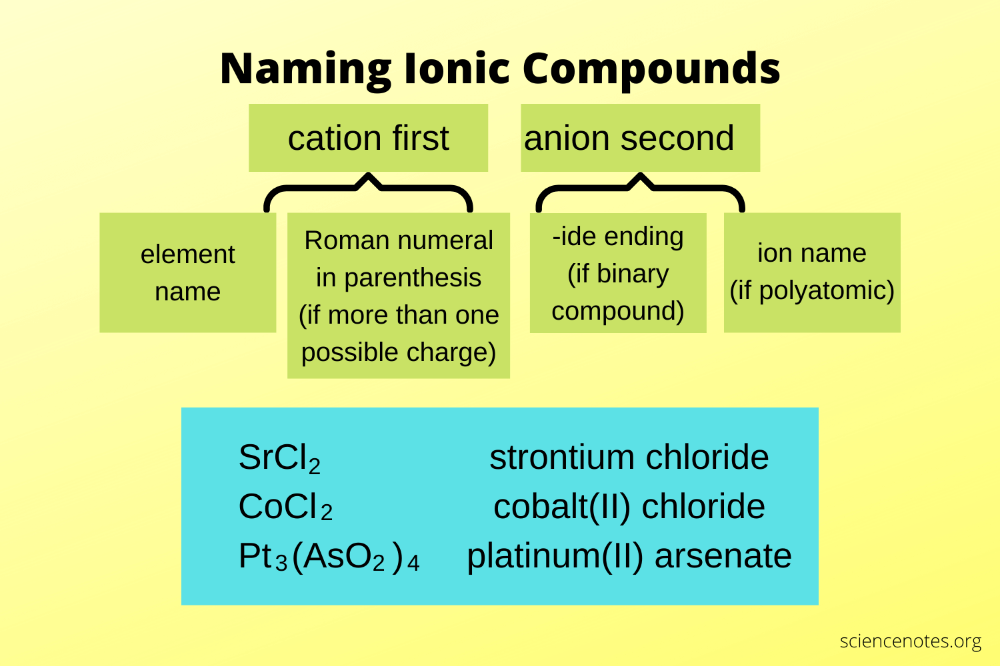
| Compound | Name | Explanation |
|---|---|---|
| NaCl | Sodium chloride | Sodium (Na) is a Group 1 metal. Chlorine (Cl) becomes chloride. |
| Fe2O3 | Iron(III) oxide | Two iron atoms have a combined charge of +6, so each Fe is +3. |
| CuSO4 | Copper(II) sulfate | Copper(II) refers to Cu2+ charge, and sulfate remains sulfate. |
| KNO3 | Potassium nitrate | Potassium (K) has a +1 charge, nitrate remains nitrate. |

Now, let's go through a step-by-step method for naming ionic compounds:
- Identify the cation and anion.
- Name the cation first. If it's a metal with variable charge, use Roman numerals.
- Name the anion, changing the ending to "-ide" for simple anions or keeping the name for polyatomic ions.
- Check the charges to ensure they balance out, which can help in confirming your compound name.
Common Mistakes and How to Avoid Them

✍️ Note: Pay attention to transition metals, which often have more than one common charge.
- Confusing Roman numerals: Use Roman numerals for transition metals or metals with variable charges.
- Not balancing charges: Make sure the total positive and negative charges equal zero.
- Incorrect anion endings: Non-metals must always end in "-ide" when simple ions.
Applications in Chemistry
Naming ionic compounds isn't just academic; it has practical applications:
- Chemical reactions: Predicting how substances will interact.
- Industry: Knowing the properties and uses of different compounds.
- Environmental science: Understanding how pollutants or salts affect ecosystems.
As we wrap up, remember that proficiency in naming ionic compounds enriches your understanding of chemistry's language. Through systematic practice using worksheets and understanding the principles behind the names, you can confidently tackle even the most complex compounds. By exploring the patterns and rules, you unlock a world where every formula tells a story of atoms bonding together. Keep practicing, referring to this guide, and soon, naming compounds will become as natural as speaking your own language.
Why do we use Roman numerals in naming some ionic compounds?

+
Roman numerals are used when the metal has multiple possible charges. They help clarify which ion is being used in the compound to balance the charges correctly.
How do you know the charge of an ion from the periodic table?
+
Main group elements in the periodic table often have predictable charges. For example, group 1 metals typically have a +1 charge, while group 17 non-metals often have a -1 charge. Transition metals are less predictable, and their charge must often be deduced from context or charge balance.
What’s the difference between covalent and ionic compounds?
+
Covalent compounds share electrons between atoms, while ionic compounds involve the complete transfer of electrons from metals to non-metals, forming ions that attract each other with electrostatic forces.
Can you name an ionic compound from its formula alone?

+
Yes, with the knowledge of the ion charges, you can name an ionic compound directly from its formula by applying the naming rules described above.
How can I improve my skills in naming ionic compounds?

+
Practice regularly with worksheets, memorize common polyatomic ions, understand the charges of main group and transition metals, and review your mistakes to improve your understanding and accuracy.