Covalent Bonding Worksheet Answer Key Revealed: Master Chemistry Now

Understanding covalent bonding is a cornerstone in the field of chemistry. This fundamental concept plays a crucial role in how molecules are formed and how they interact with each other. For students and enthusiasts eager to master chemistry, grasping the intricacies of covalent bonding can unlock a deeper understanding of chemical structures and reactions. This comprehensive guide will not only decode the Covalent Bonding Worksheet Answer Key but also provide insights that will enhance your chemical literacy.
What is Covalent Bonding?
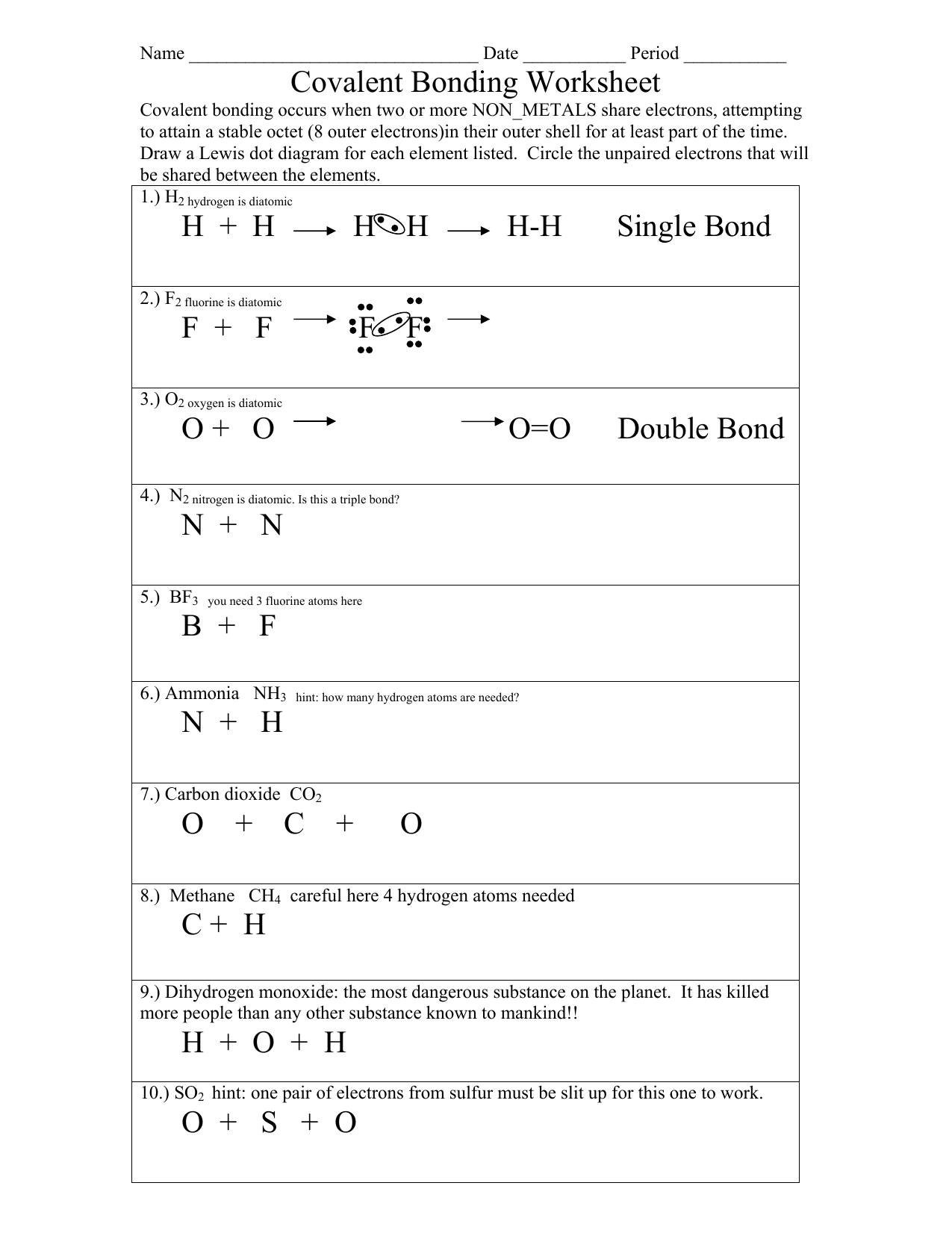
Before delving into the worksheet answer key, let’s establish a clear understanding of what covalent bonding entails:
- Definition: Covalent bonding involves the sharing of electron pairs between atoms to achieve a full outer electron shell.
- Types: There are single, double, and triple covalent bonds, each characterized by the number of shared electron pairs.
- Characteristics: Molecules with covalent bonds typically have low melting and boiling points, poor electrical conductivity, and can be polar or non-polar based on the bond's symmetry and the electronegativity of the atoms involved.

Covalent Bonding Worksheet Answer Key

Now, let’s explore the answers to common covalent bonding worksheet questions:
| Question | Answer |
|---|---|
| 1. How many covalent bonds does Carbon usually form? | Carbon forms four covalent bonds to achieve a full outer electron shell. |
| 2. What type of bonding occurs in H2O? | Hydrogen atoms share electrons with an oxygen atom to form covalent bonds in H2O. |
| 3. Explain why covalent compounds generally have lower melting and boiling points than ionic compounds. | Covalent compounds have weaker intermolecular forces (London dispersion, dipole-dipole) compared to ionic compounds which have strong ionic bonds. |
| 4. Draw the Lewis structure for CO2. |
O=C=O
|
| 5. What is the polarity of CO2 and why? | CO2 is non-polar because the molecule's symmetrical structure causes the bond dipoles to cancel each other out. |

⚗️ Note: When drawing Lewis structures, remember to satisfy the octet rule for most atoms, with exceptions like hydrogen and boron.
Common Mistakes in Understanding Covalent Bonds

To avoid common pitfalls in mastering covalent bonding, here are some misconceptions and corrections:
- Misconception: All covalent bonds are the same in strength.
Correction: The strength of a covalent bond depends on the electronegativity differences and bond type (single, double, triple). - Misconception: Covalent compounds do not conduct electricity.
Correction: While this is generally true for covalent substances in solid state, some, like electrolytes, can conduct electricity when dissolved in water. - Misconception: Hydrogen bonds are covalent bonds.
Correction: Hydrogen bonds are intermolecular forces, not covalent bonds, formed between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom.
⚗️ Note: Understanding these distinctions is key to interpreting chemical reactions and molecular behavior accurately.
Advanced Insights into Covalent Bonding

For those aiming to delve deeper into chemistry, here are some advanced concepts:
- Polarity: The difference in electronegativity between bonded atoms can result in polar covalent bonds, leading to molecular polarity.
- Resonance: Some molecules can't be accurately represented by a single Lewis structure, so resonance structures are used to depict the molecule's true electronic structure.
- Hybridization: Valence Bond Theory explains the concept of atomic orbital hybridization, crucial for understanding molecular shapes and reactivity.
Conclusion

Mastering covalent bonding is essential for anyone seeking to truly understand chemistry. From the basic principles to complex molecular structures, the information provided in this guide should give you a firm grasp on how atoms share electrons to form stable compounds. By analyzing common questions, addressing misconceptions, and exploring advanced insights, you’ve taken significant steps towards chemical proficiency. With this knowledge, you’re better equipped to handle more challenging concepts and to apply your understanding in practical situations, whether in academic studies, research, or in industrial applications.
What is the difference between a covalent and an ionic bond?

+
Covalent bonds involve the sharing of electrons to achieve a stable electron configuration, while ionic bonds are formed by the complete transfer of electrons from one atom to another, creating positive and negative ions that are electrostatically attracted to each other.
How can I determine if a molecule will be polar or non-polar?

+
Determine the molecule’s shape and the polarity of the individual bonds. If the bond dipoles do not cancel out due to molecular symmetry, the molecule is polar; otherwise, it’s non-polar.
Why do some molecules have resonance structures?

+
Resonance occurs in molecules where electrons are delocalized, and no single Lewis structure accurately represents the molecule. Instead, resonance structures collectively show the true electronic nature of the molecule.