5 Charles Law Examples for Easy Understanding

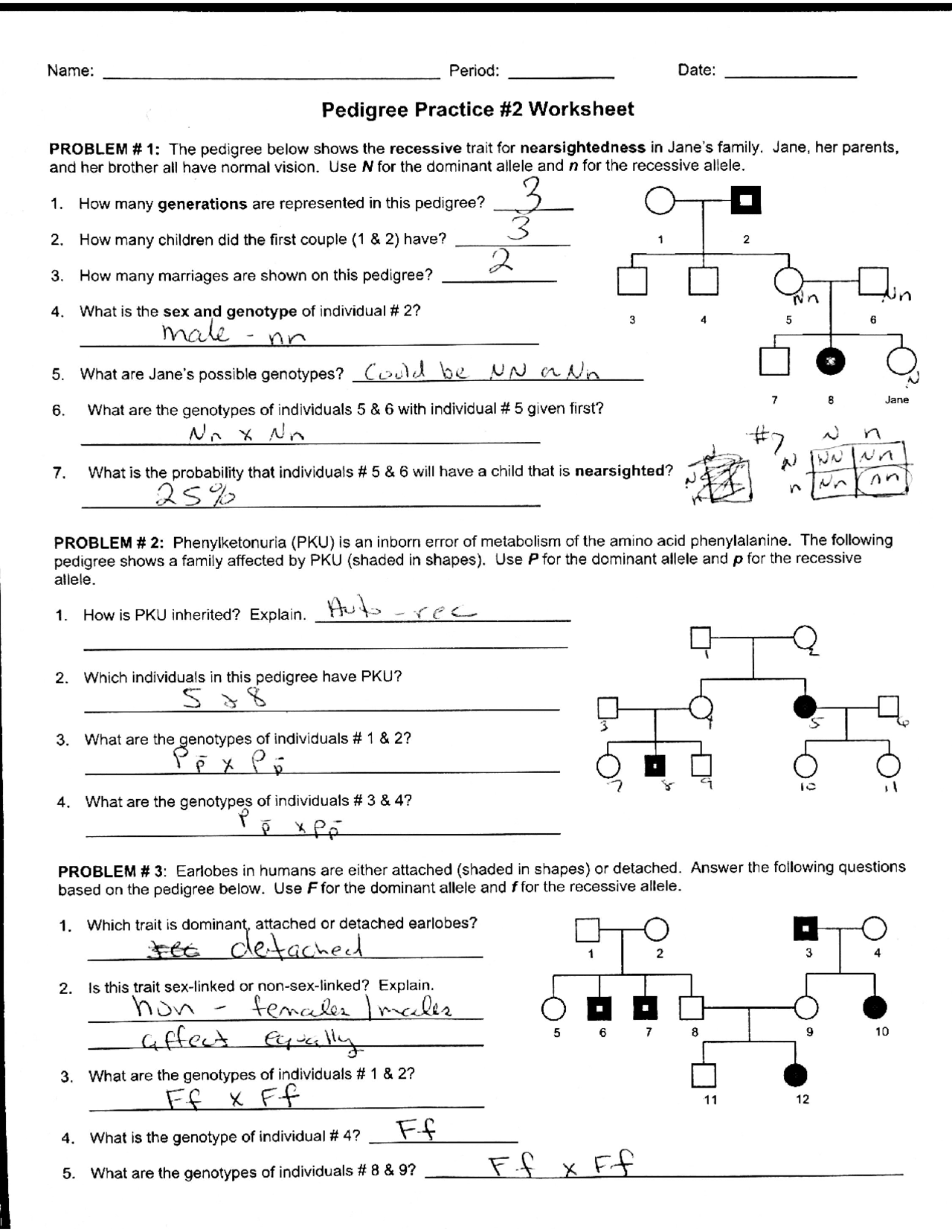
Charles's Law, named after the French scientist Jacques Charles, is one of the fundamental principles in the study of thermodynamics and gases. This law relates the volume and temperature of a gas, assuming pressure and amount of gas remain constant. Here's an explanation of Charles's Law along with practical examples to help illustrate this concept for better understanding:
What is Charles’s Law?

Charles’s Law states that for a given mass of gas at constant pressure, the volume is directly proportional to the absolute temperature. The formula for Charles’s Law is:
- V ∝ T
- Or, V = kT, where:
- V is the volume of the gas.
- T is the temperature in Kelvin.
- k is a constant.
Example 1: The Balloon in the Sun
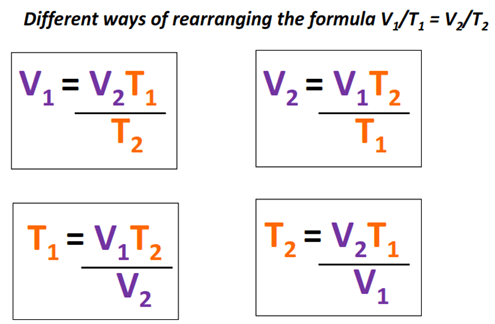
Imagine inflating a balloon with helium and placing it in direct sunlight:
- The balloon expands when the temperature increases due to the heat from the sun.
- This happens because, as per Charles’s Law, an increase in temperature (T) results in an increase in volume (V) while the pressure remains constant.
🌞 Note: Always be cautious when exposing balloons or any other gas containers to direct sunlight to avoid over-expansion and potential bursting.
Example 2: Baking Bread

When baking bread, the yeast inside the dough ferments, producing carbon dioxide gas:
- As the oven heats the dough, the gas produced expands. This expansion causes the bread to rise.
- This phenomenon follows Charles’s Law, as the increased temperature allows the gas to take up more volume, pushing the bread to rise.
Example 3: Hot Air Balloons

Hot air balloons operate on the principle of Charles’s Law:
- When air inside the balloon is heated, its volume expands, reducing its density compared to the cooler air outside.
- This decrease in density causes the balloon to lift off as it displaces heavier, cooler air.
Example 4: The Experiment with Sealed Syringe

An experiment involving a sealed syringe filled with air can demonstrate Charles’s Law:
- As you heat the syringe, the air inside expands, pushing the plunger outward.
- Upon cooling, the air contracts, pulling the plunger back in, if the syringe is allowed to return to room temperature.
🔧 Note: This experiment works best if you ensure the syringe is sealed tightly to avoid gas leakage.
Example 5: Car Tire Pressure in Winter

The pressure in car tires can be influenced by temperature changes:
- During colder months, tire pressure often drops because cold air contracts. Here, while volume inside the tire remains constant, the drop in temperature results in a lower pressure, but Charles’s Law principles still apply if we consider an idealized scenario where pressure could change:
Applying Charles’s Law in Real Life

Understanding Charles’s Law has several practical applications:
- Cooking: Why soufflés rise, or how to prevent food from expanding too much when canning.
- Engineering: Designing systems where gas expansion or contraction must be considered, like HVAC systems or refrigeration.
- Meteorology: Predicting changes in atmospheric pressure and volume based on temperature changes.
Charles's Law is a vital tool for understanding how temperature affects the volume of gases, making it essential in many fields. Its straightforward principle provides insight into everyday phenomena and industrial applications alike. By recognizing how a change in temperature can alter gas behavior, we can better control environments, design efficient systems, and make everyday tasks simpler.
The insights from Charles's Law also extend to the study of other gas laws, like Boyle's Law and the Combined Gas Law, enhancing our comprehension of gas dynamics under various conditions. Remember, always keep in mind the temperature is on the absolute scale (Kelvin), not on Celsius or Fahrenheit scales when applying Charles's Law, as the relationship fails to hold when temperature drops below absolute zero.
Does Charles’s Law apply to any gas?

+
Yes, Charles’s Law applies to any ideal gas. However, real gases approximate ideal behavior under conditions of low pressure and high temperature.
Why must the temperature be in Kelvin?
+
The Kelvin scale starts at absolute zero, where the thermal energy of the gas molecules is zero, making it the absolute temperature scale necessary for accurate calculations in gas laws.
How does Charles’s Law relate to weather balloons?

+
Weather balloons expand as they ascend due to the decreasing atmospheric pressure and increasing altitude, which reduces the surrounding air pressure while temperature changes also influence the gas inside following Charles’s Law.