5 Key Answers to Bronsted Lowry Worksheet Revealed

The concepts of acids and bases are foundational in the study of chemistry. With the Bronsted-Lowry definition, we delve deeper into the behavior of substances in chemical reactions. Here's an exploration of five key answers from a Bronsted Lowry worksheet that will not only enhance your understanding but also make you confident in tackling acid-base problems.
Understanding Bronsted Lowry Theory

The Bronsted-Lowry theory extends the definition of acids and bases from the Arrhenius model. It states:
- Acids are proton (H+) donors, and
- Bases are proton (H+) acceptors.
Here's how we typically represent these reactions:
ACID + BASE → CONJUGATE BASE + CONJUGATE ACID
This gives rise to conjugate acid-base pairs, where the conjugate base is what remains after an acid donates a proton, and the conjugate acid is what is formed when a base accepts a proton.
Key Concept 1: Identifying Bronsted Lowry Acids and Bases
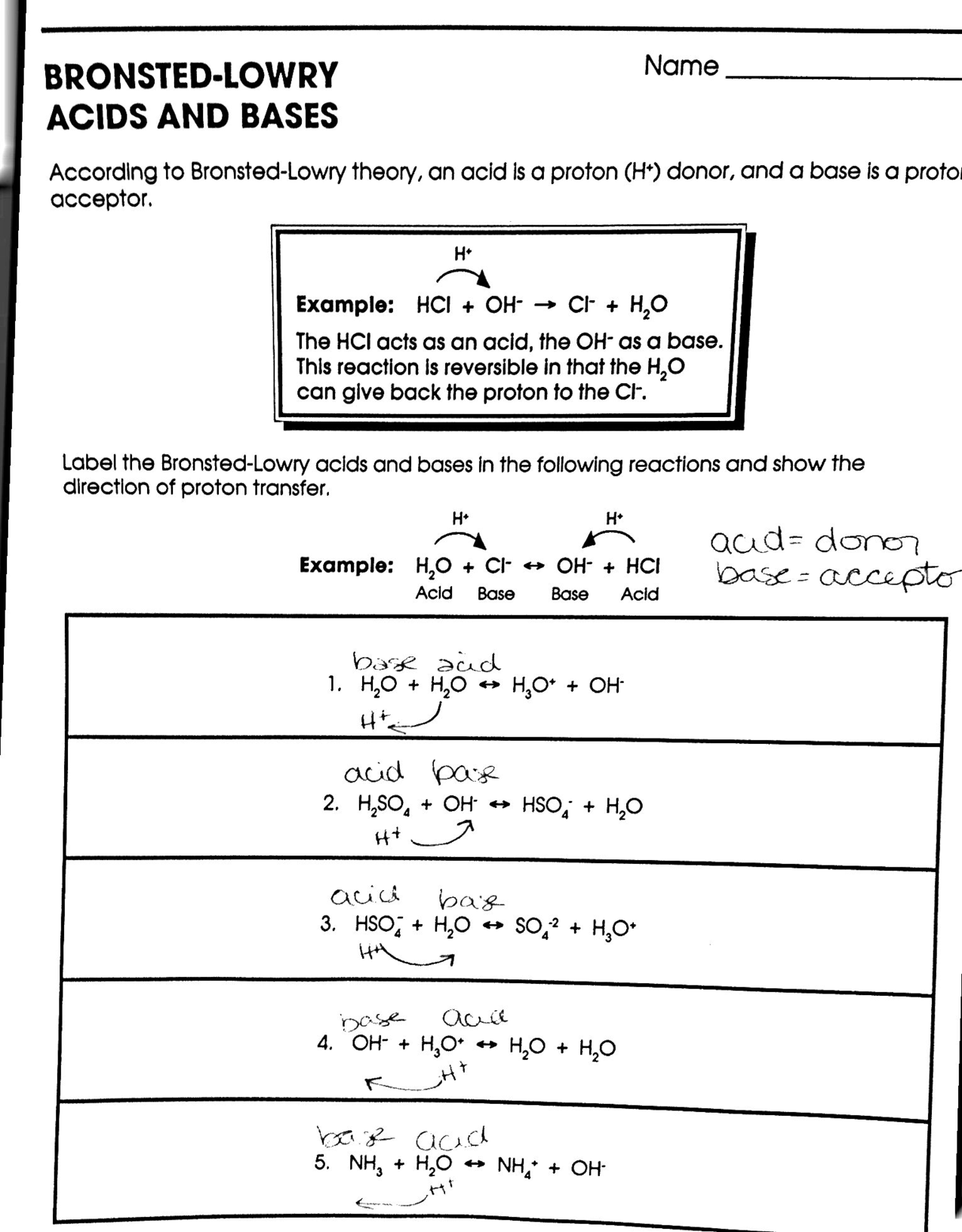
Consider a classic example:
HCl(g) + NH3(g) → NH4+(aq) + Cl-(aq)
Here, HCl is the Bronsted Lowry acid as it donates an H+ ion, while NH3 (ammonia) acts as the base accepting the proton to form NH4+.
Key Concept 2: Conjugate Acid-Base Pairs

In the reaction mentioned above:
| Acid | Conjugate Base |
|---|---|
| HCl | Cl- |
| Base | Conjugate Acid |
| NH3 | NH4+ |

Key Concept 3: Amphoteric Substances

Some substances can act both as acids and bases. Water is a prime example:
- With HCl: Water acts as a base:
HCl + H2O → H3O+ + Cl-
NH3 + H2O → NH4+ + OH-
Key Concept 4: pH and pOH

The pH scale measures the acidity of a solution. The pOH scale measures the alkalinity. Here are some key points:
- pH = -log[H3O+] and pOH = -log[OH-]
- At 25°C, pH + pOH = 14
- Lower pH values indicate stronger acids, while higher values indicate stronger bases.
Key Concept 5: Strength of Acids and Bases

The strength of an acid or base is determined by its tendency to donate or accept a proton:
- Strong Acids: Completely dissociate in water. Examples include HCl, H2SO4.
- Weak Acids: Partially dissociate in water. Examples include acetic acid (CH3COOH), formic acid (HCOOH).
- Strong Bases: Fully dissociate in water. Examples are NaOH, KOH.
- Weak Bases: Partially accept protons. Ammonia (NH3) falls under this category.
By now, you should have a firm grasp of these concepts. However, if you encounter a problem in your studies, remember that:
🔎 Note: Practice is key. Each acid-base reaction provides insight into the Bronsted Lowry model.
In understanding the Bronsted Lowry theory, you've covered significant ground in acid-base chemistry. You now know how to identify acids and bases, understand conjugate pairs, recognize amphoteric substances, interpret pH and pOH, and differentiate between strong and weak acids and bases. This knowledge is not only beneficial for academic pursuits but also for understanding real-world chemical processes, from environmental science to biological systems.
What makes a substance an acid according to Bronsted Lowry?

+
A substance is an acid if it can donate a proton (H+) to another species. This definition is more inclusive than the Arrhenius model as it applies in non-aqueous solvents as well.
Can a substance act as both an acid and a base?

+
Yes, substances like water or hydrogensulfite (HSO3-) are amphoteric, meaning they can donate or accept a proton depending on the reaction conditions.
Why is the concept of conjugate pairs important?

+
Conjugate pairs help us track the flow of protons in a reaction and understand the acid-base equilibrium. They provide a clear link between reactants and products in acid-base reactions.
How does the pH scale relate to Bronsted Lowry theory?

+
The pH scale is a measure of the hydrogen ion (H3O+) concentration, which directly correlates with the acidity of a solution. The stronger the acid, the more H3O+ it produces, and the lower the pH will be.
What are the strengths of acids and bases in the Bronsted Lowry context?

+
Strength is determined by the substance’s tendency to donate or accept protons. Strong acids fully dissociate, giving up all their protons, whereas weak acids only partially donate. The same principle applies to bases, with strong bases fully accepting protons and weak bases only partially doing so.



