Chemistry Metric Conversion: 10 Quick Worksheet Answers

Understanding and converting measurements in chemistry is not just a test of your memory, but an exercise in applying fundamental mathematical principles. In the bustling world of atoms and molecules, metric conversions are not just numbers and equations; they are the language through which we understand the fabric of the universe at a molecular level.
Why Metric Conversion Matters in Chemistry

In the realm of chemistry, precision is not just preferred; it’s essential. Whether you’re balancing an equation, determining concentration, or calculating reaction rates, every digit counts. Here are several reasons why mastering metric conversion is crucial:
- Standardization: The metric system offers a standardized approach to measurements, allowing scientists globally to communicate and work together without the confusion of different systems.
- Accuracy: It eliminates the guesswork involved in converting between non-metric units like ounces, pounds, and inches, which vary in meaning depending on the context.
- Unit Coherence: In chemistry, many laws and constants (like the gas constant) are expressed in metric units, ensuring consistency when relating different aspects of chemical science.
10 Quick Worksheet Answers for Chemistry Metric Conversion

Let’s dive into some common worksheet exercises to get your conversion skills up to par:
1. Temperature Conversion

To convert temperatures between Kelvin (K) and Celsius (°C), use the following:
- K = °C + 273.15
- °C = K - 273.15
Example: Convert 300 K to °C:
300 K - 273.15 = 26.85°C
2. Length and Distance

Here’s how to convert between commonly used length units:
| Unit | Conversion Factor to Meter |
|---|---|
| 1 Kilometer (km) | 1 km = 1000 m |
| 1 Centimeter (cm) | 1 cm = 0.01 m |
| 1 Millimeter (mm) | 1 mm = 0.001 m |

Example: Convert 100 cm to km:
100 cm * (0.01 m/1 cm) * (1 km/1000 m) = 0.001 km
3. Mass and Weight

Let's look at converting between grams, kilograms, and milligrams:
| Unit | Conversion Factor to Gram |
|---|---|
| 1 Kilogram (kg) | 1 kg = 1000 g |
| 1 Milligram (mg) | 1 mg = 0.001 g |
Example: Convert 2.5 kg to mg:
2.5 kg * (1000 g/1 kg) * (1000 mg/1 g) = 2,500,000 mg
4. Volume
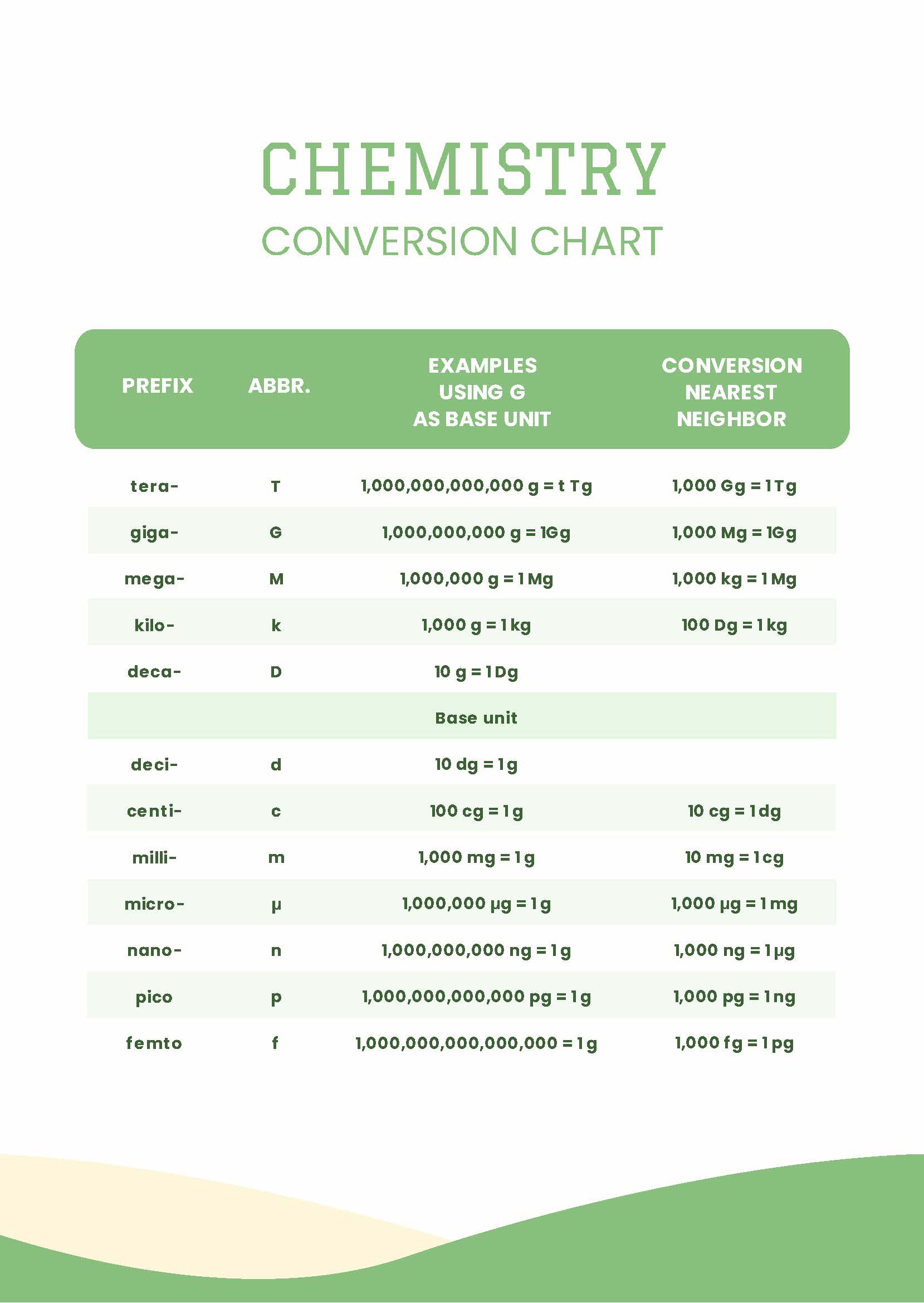
Volume conversions can seem tricky, but remember the following:
- 1 Liter (L) = 1000 milliliters (mL)
- 1 mL = 1 cubic centimeter (cm³)
Example: Convert 2.5 L to cm³:
2.5 L * (1000 mL/1 L) * (1 cm³/1 mL) = 2500 cm³
💡 Note: Always double-check your conversions with dimensional analysis to ensure accuracy in chemistry calculations.
5. Density

Density (d) is defined as mass per unit volume and can be derived from:
- d = mass/volume
Example: Calculate the density of a substance with a mass of 30 g and a volume of 15 mL:
d = (30 g)/(15 mL) = 2 g/mL
6. Molar Mass and Conversion

The molar mass of a compound is the sum of the atomic masses of its constituent elements. Here’s how to convert grams to moles using molar mass:
moles = mass (g) / molar mass (g/mol)
Example: Calculate the moles in 50 g of H2O:
Molar mass of H2O = 18.015 g/mol moles = 50 g / (18.015 g/mol) = 2.776 mol
7. Concentration and Dilution

In solutions, concentration is often expressed in molarity (M), which is moles of solute per liter of solution:
M = moles of solute / volume of solution (L)
Example: What is the molarity of a solution containing 0.5 moles of NaCl in 250 mL of solution?
M = (0.5 mol) / (0.250 L) = 2 M
8. Pressure

Pressure in chemistry is commonly measured in atmospheres (atm), pascals (Pa), or mmHg:
- 1 atm = 101,325 Pa = 760 mmHg
Example: Convert 500 mmHg to atm:
500 mmHg * (1 atm/760 mmHg) = 0.6579 atm
9. Heat and Energy

The SI unit for energy is the joule (J), but in chemistry, we often use the calorie or kilojoule:
- 1 calorie (cal) = 4.184 joules (J)
- 1 joule (J) = 0.239 calorie (cal)
Example: Convert 500 J to kcal:
500 J * (1 cal/4.184 J) * (1 kcal/1000 cal) = 0.1195 kcal
10. pH and pOH

Understanding the relationship between pH and pOH is crucial:
- pH + pOH = 14
Example: If the pH of a solution is 8.4, what is its pOH?
pOH = 14 - pH = 14 - 8.4 = 5.6
To summarize, metric conversion in chemistry is not a mere formality. It's a foundational skill that translates data into a universal language, allowing for the seamless integration of different experiments and discoveries. By mastering these conversions, you're not just learning to count; you're learning to connect, interpret, and innovate at the molecular level.
Why are metric units preferred in scientific research?
+Metric units are preferred in science for their simplicity and coherence. They follow a decimal system, making conversions straightforward. This system’s universal adoption ensures clarity and consistency across different countries and scientific disciplines.
How can I easily remember metric conversion factors?
+To remember metric conversions, understand the prefixes like kilo (k), centi ©, milli (m) etc., which denote powers of 10. Mnemonics like “King Henry Doesn’t Usually Drink Chocolate Milk” can help where each letter represents a prefix. Regular practice also aids in memorization.
What if I need to convert between different units not covered here?
+If you encounter units not discussed here, utilize conversion tables available online or consult the International System of Units (SI) for official standards. Dimensional analysis can help you derive new conversion factors logically.
What are the most common mistakes in metric conversions in chemistry?
+Common mistakes include:
- Using incorrect prefixes or conversion factors.
- Not paying attention to significant figures.
- Confusing units like grams and kilograms, or liters and milliliters.
Always double-check your work and use dimensional analysis for accuracy.
Related Terms:
- Metric conversion Worksheet PDF