Boyle's and Charles' Law Worksheet: Simple Gas Law Explanations

In the realm of physics, understanding the behavior of gases under different conditions of pressure, volume, and temperature is crucial. Boyle's Law and Charles' Law, named after their respective discoverers, provide a foundational framework for explaining how gases behave. This article will delve into these gas laws, offering clear explanations, practical examples, and helpful insights to enhance your understanding and mastery of the subject.
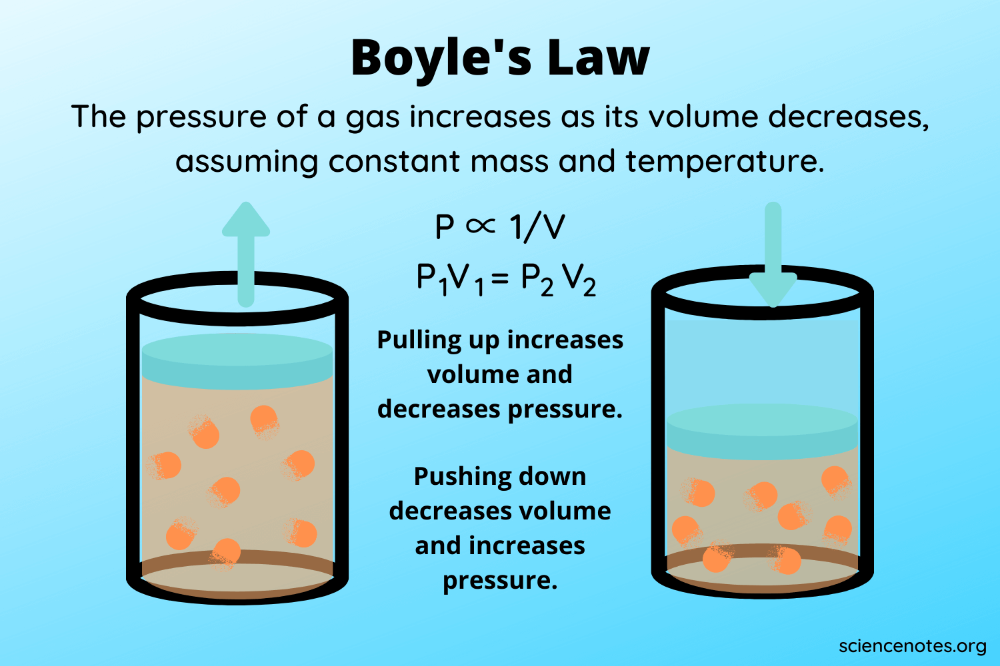
Boyle’s Law


Boyle’s Law, formulated by Robert Boyle in the mid-17th century, is a cornerstone in the study of gases. It states that:
The pressure exerted by a gas (P) is inversely proportional to its volume (V) at constant temperature.
Mathematically, this can be expressed as:
P1V1 = P2V2
- P1 and V1 represent the initial pressure and volume.
- P2 and V2 are the pressure and volume after some change has occurred.
Example of Boyle’s Law

Consider a balloon with a certain amount of gas. If you press on the balloon, the volume decreases, but the pressure increases to maintain the product of PV constant. Let’s quantify this with an example:
| Initial Condition | Final Condition |
|---|---|
| Pressure = 2 atm | Pressure = 4 atm |
| Volume = 10 L | Volume = ? |

Using Boyle's Law:
P1V1 = P2V2
(2 atm)(10 L) = (4 atm)(V2)
V2 = 5 L
⚠️ Note: The inverse relationship between pressure and volume can be counterintuitive but is a fundamental principle of gas behavior.
Charles’ Law


Jacques Charles, a French scientist, discovered that the volume of a gas is directly proportional to its absolute temperature at constant pressure. This relationship, known as Charles’ Law, can be described mathematically as:
V1/T1 = V2/T2
- V1 and T1 are the initial volume and temperature (in Kelvin).
- V2 and T2 are the final volume and temperature (in Kelvin).
Example of Charles’ Law
Imagine a gas cylinder with an initial volume of 250 mL at a temperature of 25°C (298 K). If the temperature is increased to 50°C (323 K), what would be the new volume?
V1/T1 = V2/T2
(250 mL)/(298 K) = (V2)/(323 K)
V2 ≈ 271 mL
☝ Note: Always remember to use absolute temperature in Kelvin when applying Charles' Law.
Combining Boyle’s and Charles’ Laws

The Gas Law Worksheet would not be complete without combining these fundamental laws. The Ideal Gas Law, which combines Boyle’s and Charles’ Laws along with Avogadro’s Law, is expressed as:
PV = nRT
- P = Pressure
- V = Volume
- n = Number of moles of gas
- R = Gas constant (0.0821 L·atm·mol-1·K-1)
- T = Absolute temperature (in Kelvin)
This equation provides a comprehensive view of how changes in pressure, volume, temperature, and the amount of gas affect each other. While beyond our main scope, it's worth noting for context.
Summing Up

Understanding Boyle’s and Charles’ Laws not only helps you predict the behavior of gases but also equips you with the foundational knowledge necessary for more advanced study in thermodynamics, meteorology, chemistry, and numerous engineering disciplines. The ability to conceptualize and apply these laws in real-world scenarios, from the expansion of a balloon to the operation of a gas cylinder, underpins our understanding of the dynamic interactions between different states of matter.
What is the difference between Boyle’s Law and Charles’ Law?

+
Boyle’s Law relates to the inverse relationship between pressure and volume at a constant temperature, while Charles’ Law deals with the direct relationship between volume and temperature at constant pressure.
Can Boyle’s and Charles’ Laws be used for all gases?

+
These laws are approximations that work best for ideal gases or at low pressures and moderate temperatures where real gases behave most like ideal gases.
How do these laws relate to everyday life?

+
Everyday applications include inflating balloons, explaining why a tire’s pressure increases with rising temperatures, and understanding the functioning of aerosol sprays.