Bill Nye Phases of Matter Worksheet Answers Revealed

Introduction to Phases of Matter

Bill Nye, popularly known as ‘The Science Guy,’ has a remarkable ability to make science engaging, especially for younger audiences. His episode on the phases of matter is both informative and entertaining, making complex scientific concepts accessible. This blog post delves into the phases of matter, exploring the various states of matter and providing comprehensive Bill Nye Phases of Matter Worksheet Answers to aid in educational endeavors.

What Are the Phases of Matter?
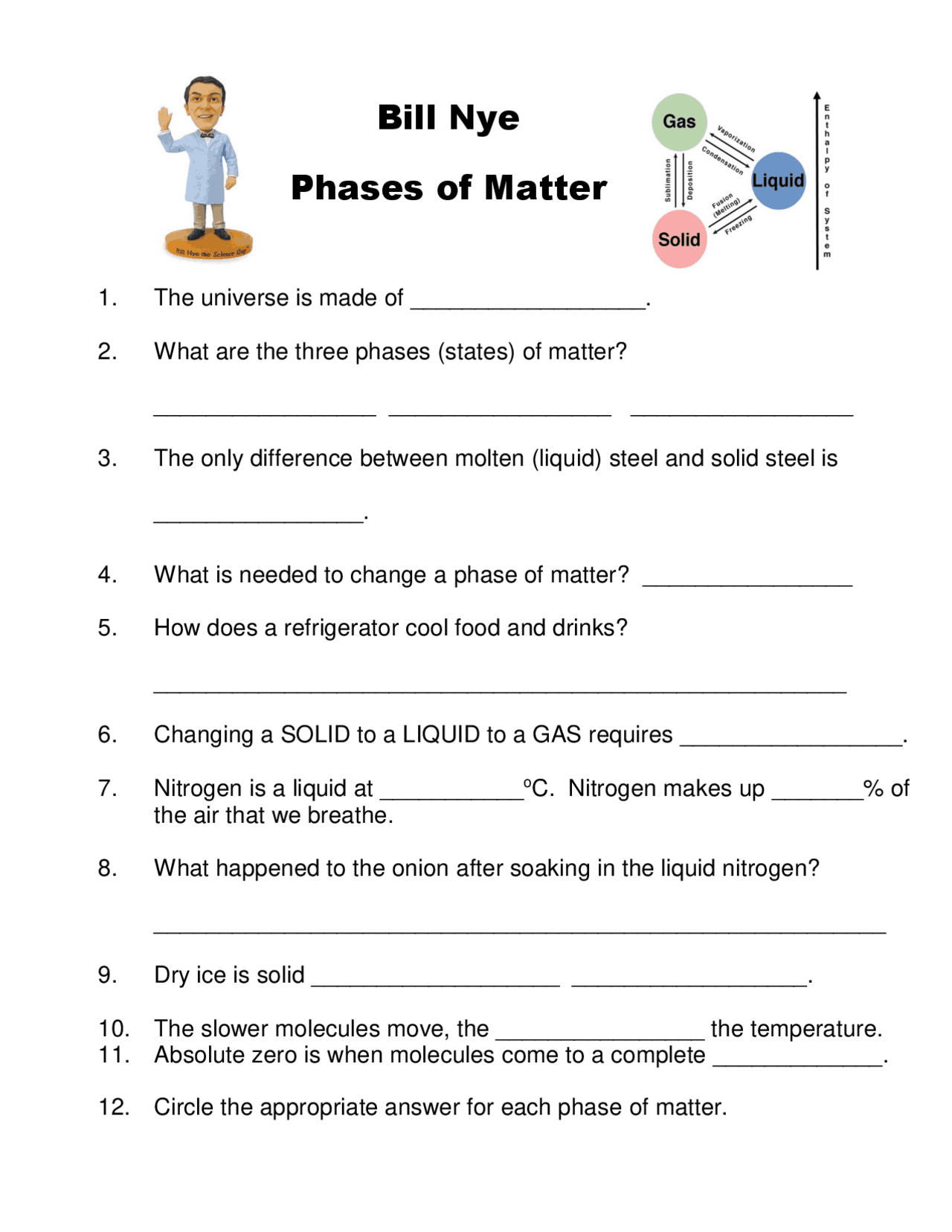
Matter exists in several phases, but we generally focus on the most common three: solid, liquid, and gas. Here’s a closer look at each:
- Solid: In this phase, particles are closely packed together, often in a regular pattern, with limited mobility. Solids have a fixed shape and volume.
- Liquid: Liquid particles have more mobility than those in solids. They move around but remain close, giving liquids a fixed volume but no fixed shape.
- Gas: Particles in a gas move freely and independently, far apart from each other, which results in gas having no fixed shape or volume.
🧪 Note: There are also other phases of matter like plasma, Bose-Einstein condensate, and neutronium, but these are less common in everyday experiences.
Exploring the Properties

Each phase of matter has unique properties that define it:
- Compressibility: Gases are highly compressible; solids and liquids much less so.
- Temperature Sensitivity: Changes in temperature can change the phase of matter.
- Volume and Shape: Solids have a definite shape and volume, liquids have a definite volume but adapt to their container’s shape, and gases take the shape and volume of the container.
| Phase | Shape | Volume | Compressibility | Energy |
|---|---|---|---|---|
| Solid | Definite | Definite | Very Low | Lowest |
| Liquid | Indefinite | Definite | Low | Medium |
| Gas | Indefinite | Indefinite | High | Highest |

Worksheet Answers

Here are some potential questions and answers that might appear in a Bill Nye Phases of Matter Worksheet:
Why Can Water Be Solid, Liquid, and Gas?

Water can exist in all three phases because its melting and boiling points are relatively close together in terms of temperature on Earth. This means that with slight changes in temperature, water can shift from one phase to another.
💧 Note: The triple point of water, where all three phases coexist, occurs at 0.01°C and 611.657 Pa.
What Happens When Matter Changes Phase?

When matter changes phase, it either absorbs or releases energy. Here’s a breakdown:
- From Solid to Liquid (Melting): Matter absorbs heat, and the solid’s molecular bonds weaken, allowing the molecules to move more freely.
- From Liquid to Gas (Evaporation): Energy is absorbed as the bonds break completely, turning the liquid into vapor or gas.
- From Gas to Liquid (Condensation): Energy is released when gas particles lose enough energy to come closer together, forming liquid.
- From Liquid to Solid (Freezing): Energy is released, molecules slow down, and stronger bonds form.
What Are Some Examples of Each Phase?

Here are a few examples:
- Solid: Ice, rock, wood
- Liquid: Water, oil, syrup
- Gas: Oxygen, helium, steam
🌡 Note: Phases can change at different temperatures depending on atmospheric pressure.
The exploration of the phases of matter through the engaging narrative of Bill Nye makes these concepts not only educational but also memorable. Understanding these fundamental properties helps us appreciate the world around us and forms the basis for many advanced scientific explorations.
In summary, this blog post has covered the basic phases of matter—solid, liquid, and gas—and how these phases are influenced by temperature, energy, and pressure. We've also provided comprehensive answers to common worksheet questions, aiding students in mastering this essential part of physical science. By studying these phases, we not only learn about the tangible aspects of matter but also unlock deeper insights into how our universe functions at its core.
What makes a substance a solid?

+
Particles in a solid are closely packed, often in a fixed pattern, with very limited motion, which gives solids their rigidity and definite shape and volume.
Can a substance exist in more than one phase at once?

+
Yes, at specific conditions like the triple point, a substance can exist in all three phases simultaneously.
Why is plasma considered a phase of matter?

+
Plasma, though less common on Earth, is the most abundant phase in the universe. It is an ionized gas consisting of charged particles that exhibit unique behaviors, like responding to magnetic fields, making it distinct from other phases.