Mole Calculations: 5 Expert Tips for Worksheet Mastery

Mastering mole calculations is crucial for students studying chemistry, especially when navigating through the complex world of chemical quantities. The concept of a mole is fundamental because it allows chemologists to count atoms, molecules, or ions by weighing them. This blog post will walk you through five expert tips designed to help you conquer mole calculations and excel in your worksheets. From understanding the basics to advanced troubleshooting, we'll provide insights and methods to improve your skills.
The Mole: Understanding the Basics

Before diving into calculations, let’s establish a foundational understanding:
- Mole: Defined as the amount of substance containing as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. It’s Avogadro’s number (6.022 x 10^23) of entities.
- Formula Weight: The sum of the atomic weights of all atoms in a molecule.
- Molar Mass: The mass of one mole of a substance, often in grams per mole.
Tip 1: Use Dimensional Analysis to Simplify Mole Problems

Dimensional analysis, or the factor-label method, helps eliminate unit conversion errors:
- Start with what you know, and write the conversion factors in the form of fractions.
- Ensure units cancel out sequentially to arrive at the desired unit.
| Given Value | Conversion Factor | Desired Unit |
|---|---|---|
| 58.44g | 1 mole NaCl/58.44g | moles |
| 1.5 moles | 6.022 x 10^23 molecules/1 mole | molecules |

Tip 2: Master Molarity Calculations

Molarity is the number of moles of solute per liter of solution:
- Formula: Molarity (M) = moles of solute / volume of solution in liters (L)
- Remember that the volume must be in liters, and moles are determined by using the substance’s molar mass.
Tip 3: Utilize Stoichiometry in Balancing Equations

Stoichiometry deals with the quantitative relationships in chemical reactions:
- Use mole ratios derived from balanced equations to convert moles of one substance into moles of another.
- Ensure all coefficients in the balanced equation are considered for mole-to-mole conversion.
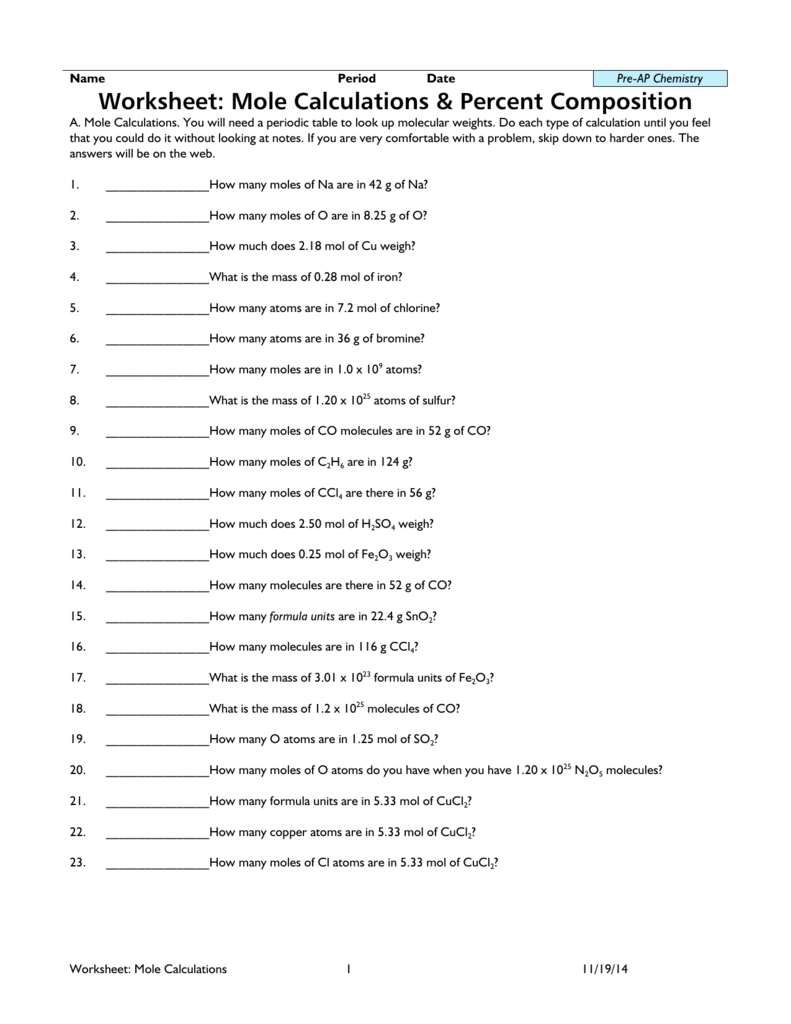
Tip 4: Practice, Practice, Practice
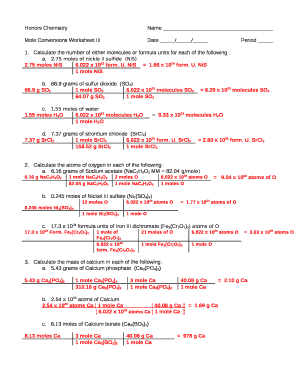
The key to mastering mole calculations:
- Work through as many worksheets and practice problems as possible.
- Focus on different types of problems to understand various scenarios (mass-to-mole, mole-to-mass, and mole-to-mole conversions).
Tip 5: Troubleshooting Common Mistakes

Here are some common pitfalls and how to avoid them:
- Unit Errors: Always keep track of your units, and remember that 1000 mL = 1 L.
- Rounding: Avoid premature rounding; always carry out intermediate calculations to at least three significant figures.
- Conversion Errors: Double-check your dimensional analysis to ensure unit cancellation leads you to the correct final unit.
- Reaction Conditions: Be mindful of reaction conditions that might influence molar volume or solubility.
💡 Note: Proper dimensional analysis significantly reduces errors in mole calculations, fostering a better understanding of chemical concepts.
In wrapping up, mastering mole calculations involves a blend of understanding core principles, applying them effectively, and consistent practice. By using dimensional analysis, understanding molarity, leveraging stoichiometry, committing to practice, and avoiding common errors, you’ll enhance your problem-solving prowess in chemistry. This proficiency not only aids in academic pursuits but also lays a solid foundation for practical applications in chemistry-related careers.
What is a mole in chemistry?

+
A mole is a unit of measurement in chemistry used to express amounts of a chemical substance, defining 6.022 x 10^23 entities (atoms, molecules, ions, or electrons) of that substance.
Why is the mole important?
+
The mole is crucial because it links the mass of a substance to the number of its particles, enabling chemists to perform accurate chemical reactions and understand material properties on a micro and macro scale.
How do I convert grams to moles?

+
To convert grams to moles, divide the mass of the substance in grams by its molar mass (in grams per mole). The formula is: moles = mass / molar mass.
What are some common mistakes in mole calculations?

+
Common mistakes include unit conversion errors, premature rounding, not balancing equations correctly, and misunderstanding the stoichiometry of reactions.



