5 Tips for Solving Balancing Equations Worksheet Easily

If you've ever struggled with balancing chemical equations or found yourself stuck on a balancing equations worksheet, you're not alone. Chemical equations are fundamental in chemistry, as they provide a precise representation of chemical reactions. Whether you're a student, a teacher, or simply someone revisiting chemistry, mastering the art of balancing equations can seem daunting at first, but with the right approach, it can become second nature. Here are five tips to help you solve balancing equations worksheets with ease:
Understand the Concept

Before you dive into solving equations, it’s critical to understand what balancing actually means:
- Conservation of Mass: In a balanced equation, the number of atoms of each element on both sides of the reaction arrow remains the same, adhering to the Law of Conservation of Mass.
- Chemical Entities: Molecules, atoms, ions, or other species involved in the reaction must be balanced to reflect their real interactions in nature.
Use the Inspection Method

The most straightforward approach to balancing equations is the inspection method, also known as the hit-and-trial method. Here’s how you can utilize it:
- Start with the simplest element: Choose the element that appears in only one reactant and one product. Balance this element first.
- Adjust coefficients: Change the coefficient in front of the compound to balance this element. Do not alter subscripts as this would change the compound itself.
- Work through the other elements: Continue with other elements, adjusting coefficients as needed, until all elements are balanced. This might require you to revisit previously balanced elements due to their interconnected nature.
🔍 Note: You might find that using a method like the oxidation number method or ion-electron method is more appropriate for complex redox reactions.
Check Your Work with Formulas

Double-checking your work is crucial:
- Atom Count: Ensure the number of atoms of each element is the same on both sides of the equation.
- Charge Balance: For ionic reactions, make sure the net charge is balanced.
- Validate Ratios: The stoichiometric coefficients should reflect the actual ratio of compounds reacting in the real world.
Practice with Different Scenarios
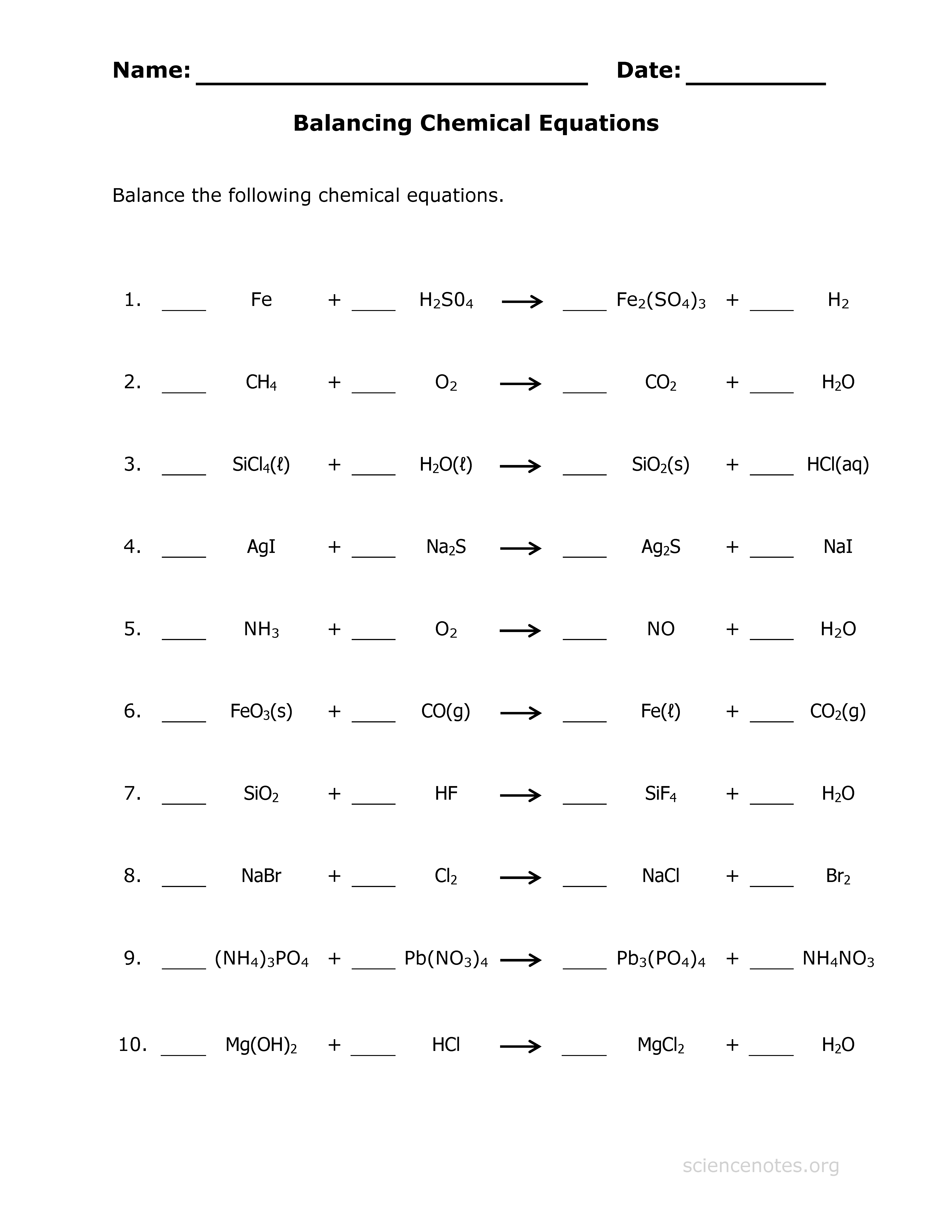
Practicing with a variety of scenarios can significantly improve your ability to balance equations:
- Simple Decomposition Reactions: Where a compound breaks down into simpler substances.
- Combination Reactions: Where two or more elements or compounds react to form a single product.
- Combustion Reactions: Where a compound reacts with oxygen to form oxides.
- Redox Reactions: Practice balancing equations for reactions involving oxidation and reduction.
🧪 Note: Balancing redox reactions can be more complex due to the need to consider electron transfer, but practice can lead to proficiency.
Utilize Tools and Software

In today’s digital age, balancing equations can be made easier with:
- Equation Balancers: Online tools like Chembalancer or other balancing calculators can instantly provide solutions or verify your own work.
- Educational Apps: Numerous apps exist to help students learn and practice balancing equations interactively.
- Chemistry Software: Advanced software can not only balance equations but also simulate reactions and predict outcomes.
In summary, mastering the art of balancing chemical equations involves a blend of understanding fundamental concepts, employing practical methods like the inspection technique, double-checking your work, practicing with diverse reactions, and using available tools to enhance your learning experience. Remember, proficiency in balancing equations is not just about solving a worksheet; it’s about comprehending the chemistry behind the reactions you’re describing. As you continue to balance more equations, you'll develop an intuitive sense of how substances react with one another, making the process increasingly effortless. Let's embrace this challenge as an opportunity to deepen our understanding of chemistry.
Why is balancing chemical equations important?

+
Balancing chemical equations ensures that the law of conservation of mass is followed, providing an accurate depiction of how reactants transform into products, which is essential for understanding the stoichiometry of reactions and predicting outcomes in chemistry.
Can balancing chemical equations be learned through practice alone?

+
Yes, practice is key to mastering balancing equations. While understanding the concept is vital, continuous practice through worksheets and diverse reaction types will enhance your ability to balance equations effectively.
What should I do if the equation seems unbalanced?

+
If you find the equation difficult to balance, start with the simpler elements first. Sometimes, revisiting previously balanced elements might be necessary due to the interdependence of coefficients. If needed, use balancing tools to verify your work.
Is it okay to change the subscripts in a chemical formula to balance an equation?

+
No, changing subscripts in a chemical formula alters the compound itself, which is incorrect. You should only adjust the coefficients in front of the compounds to balance the equation while maintaining the chemical identity of each substance involved.