5 Essential Tips for Moles Molecules and Grams

Chemistry can often seem like a language of its own, with its unique lexicon of moles, molecules, and grams, each holding a specific place in the structure of chemical calculations. Understanding these concepts is not just fundamental for budding chemists or students of the subject but is crucial for anyone dabbling in scientific studies or related fields. This comprehensive guide aims to demystify these concepts, offering practical tips to master the interconversion between moles, molecules, and grams.
Understanding Moles, Molecules, and Grams

Before diving into the tips, it’s essential to establish a clear understanding of what moles, molecules, and grams represent in chemistry:
- Moles: A mole is a unit of measurement for the amount of substance, defined as 6.022 x 1023 particles (atoms, molecules, ions, etc.). This number, known as Avogadro’s number, is like a universal translator for scaling up the very small world of atoms and molecules to amounts we can more easily measure.
- Molecules: These are groups of atoms bonded together, representing the smallest fundamental unit of a chemical compound that has the chemical properties of that compound.
- Grams: In chemistry, grams are used to measure the mass of a substance, providing a tangible way to quantify chemicals in our macroscopic world.
Tip 1: Master the Molar Mass

The key to converting between moles and grams is the molar mass of a substance:
- Calculate molar mass by summing the atomic masses of all atoms in a molecule or a formula unit. For example, for H2O, you would add the atomic mass of two hydrogen atoms (2 x 1.008 g/mol) and one oxygen atom (16.00 g/mol) to get 18.016 g/mol.
🔬 Note: Atomic masses are usually rounded to two decimal places for simplicity.
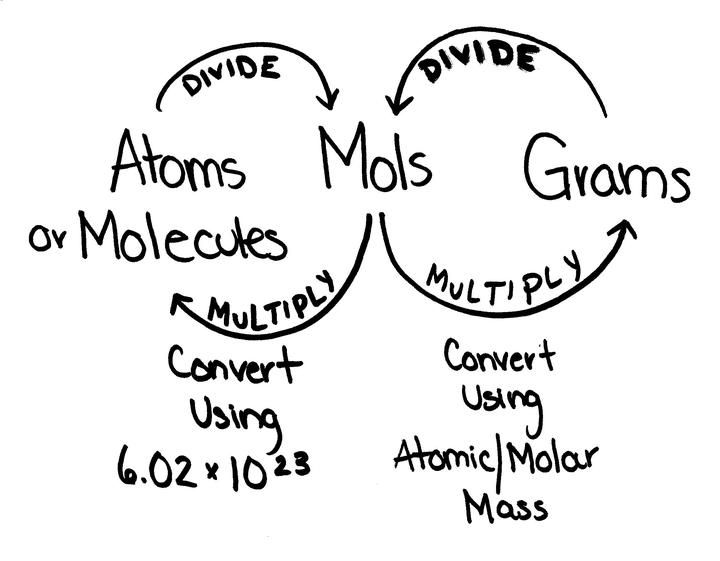
Tip 2: Use Avogadro’s Number for Mole to Molecule Conversion
Converting moles to molecules (or vice versa) involves using Avogadro’s number:
- Multiply the number of moles by 6.022 x 1023 to get the number of molecules.
- Conversely, divide the number of molecules by 6.022 x 1023 to find the number of moles.
Tip 3: Grams to Moles - Use Molar Mass

To convert grams to moles or moles to grams:
- Divide the mass in grams by the molar mass to get moles.
- Multiply moles by the molar mass to convert back to grams.
🔢 Note: Remember, the molar mass is substance-specific, so always use the correct value for your calculations.
Tip 4: Apply Dimensional Analysis

Dimensional analysis, or the factor-label method, is an excellent tool for these conversions:
- Write down what you have (e.g., 50 grams of NaCl) and where you want to go (e.g., moles of NaCl).
- Use conversion factors derived from definitions (like molar mass, Avogadro’s number) to bridge the units, ensuring all units cancel out, leaving you with the desired unit.
Tip 5: Practice with Real-World Examples
Applying these concepts to real-world scenarios solidifies your understanding:
- Calculate the amount of sodium carbonate you need to make a certain volume of a solution.
- Estimate how many molecules are in a drop of water.
Here’s a table that summarizes some common conversions:
| Conversion | Formula |
|---|---|
| Grams to Moles | Grams / Molar Mass |
| Moles to Molecules | Moles * 6.022 x 1023 |
| Molecules to Moles | Molecules / 6.022 x 1023 |

By mastering these tips, you not only enhance your understanding of chemistry but also equip yourself with the tools to solve complex problems in stoichiometry, analytical chemistry, and beyond. Remember, these conversions are not just academic exercises; they are practical tools used daily in laboratories around the world.
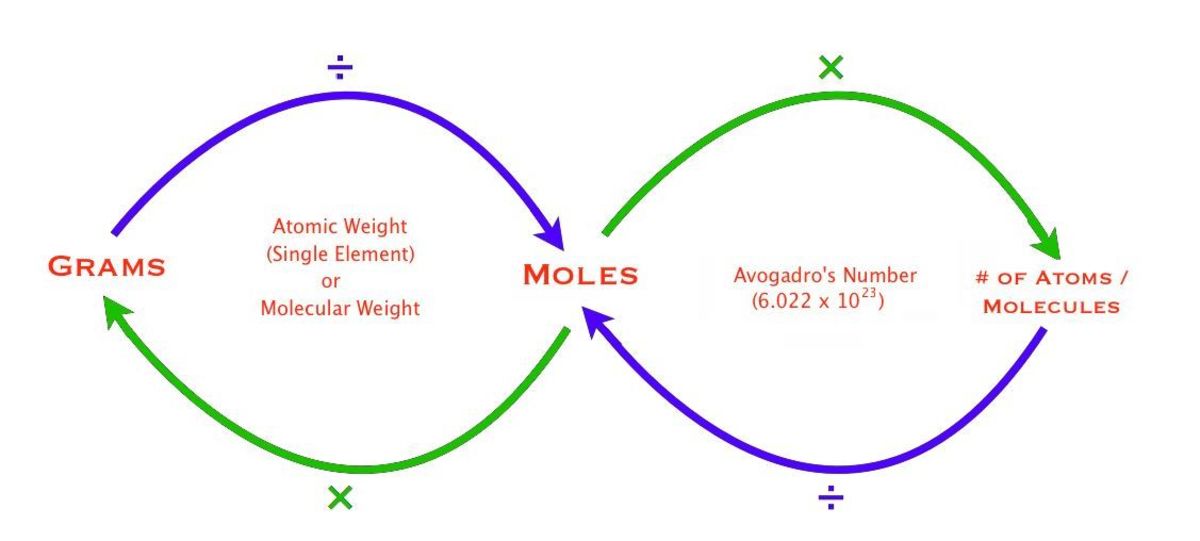
Why is Avogadro’s number important?
+
Avogadro’s number provides the link between the microscale world of atoms and molecules to our macroscopic observations. It tells us how many particles are in one mole of any substance.
How can I remember the formulas for these conversions?

+
Visual aids like charts or tables, regular practice, and mnemonics can help. For instance, you could remember “MAG” for Molar mass (M), Avogadro’s number (A), and Grams (G) to navigate between them.
What are some common mistakes in mole to gram conversions?

+
Common errors include using the incorrect molar mass, forgetting to account for the substance’s formula weight, and misinterpreting the units of measurement (e.g., grams versus kilograms).