5 Essential Facts About Atom Structure You Must Know

Understanding the atom structure is foundational for anyone exploring the realms of chemistry or physics. Atoms, the smallest units of matter, have a unique composition that dictates their behavior, reactivity, and how they form compounds. This blog post delves into five crucial aspects of atom structure, ensuring you grasp the fundamental principles behind this microscopic world.
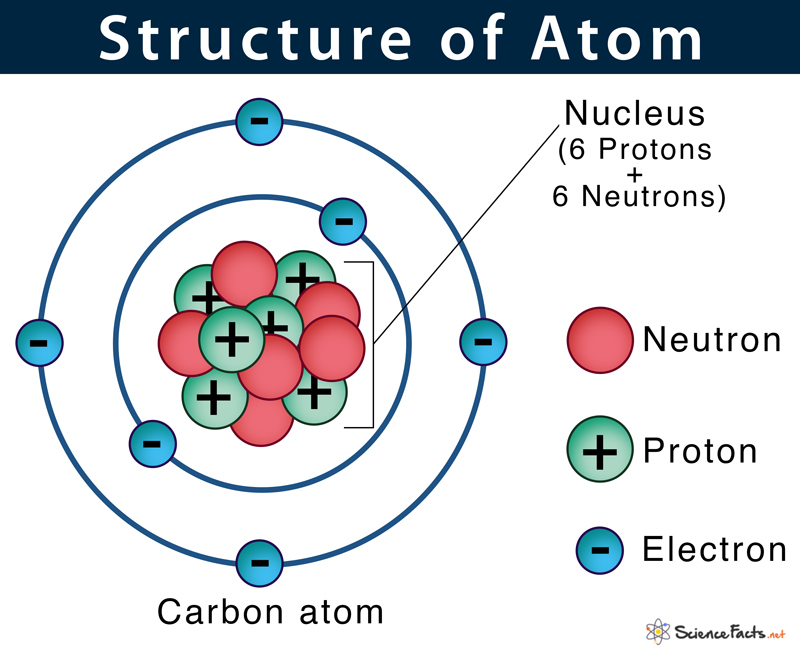
1. Components of an Atom


An atom is composed of three main subatomic particles:
- Protons: Positively charged particles found in the nucleus. The number of protons defines an element’s atomic number.
- Neutrons: Neutral particles, also located in the nucleus. The sum of protons and neutrons gives the mass number.
- Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels.
2. Electron Configuration

Electrons do not randomly hover around the nucleus; they are organized in distinct layers known as:
- Energy Levels (Shells): Represented as ‘n’, where n=1 is the closest shell to the nucleus.
- Subshells (Orbitals): Within each energy level, electrons are distributed in subshells labeled s, p, d, and f.
- Electron Shell Model: Shows how electrons are distributed in their shells, affecting the atom’s reactivity and bonding.
| Energy Level (n) | Maximum Number of Electrons |
|---|---|
| 1 | 2 |
| 2 | 8 |
| 3 | 18 |
| 4 | 32 |

💡 Note: Each orbital can hold a maximum of 2 electrons, following the Pauli Exclusion Principle.
3. Atomic Number and Mass Number

The atomic number is the number of protons in an atom’s nucleus. It determines the element’s identity. The mass number, on the other hand, is the total number of protons and neutrons. Here’s a quick way to calculate:
- Mass Number = Number of Protons + Number of Neutrons
4. Isotopes and Ions

Atoms of the same element can differ in:
- Isotopes: Atoms with the same number of protons but different numbers of neutrons. This difference affects the atom’s mass but not its chemical properties.
- Ions: When an atom gains or loses electrons, it becomes charged. Positive ions (cations) lose electrons, while negative ions (anions) gain them. The ionization process alters how atoms interact with others.
5. Nuclear Forces and Stability

Nuclear forces are responsible for the binding energy in an atom:
- Strong Nuclear Force: Holds protons and neutrons together against the electromagnetic force of protons repelling each other.
- Weak Nuclear Force: Involved in radioactive decay, where an unstable nucleus transforms into a more stable state.
🔬 Note: Understanding these forces helps predict atom stability and the behavior during nuclear reactions.
Understanding these aspects of atomic structure provides a comprehensive view of how atoms interact to form compounds, how they behave in chemical reactions, and why they exhibit certain properties. This knowledge not only enriches our understanding of the natural world but also drives innovations in various scientific fields.
What determines an element’s atomic number?

+
The atomic number is determined by the number of protons in an atom’s nucleus.
Can electrons exist in any energy level?

+
Electrons can only exist in discrete energy levels. They can move between levels by absorbing or emitting energy.
What makes an isotope of an element different from its standard form?
+
Isotopes have the same number of protons but differ in their number of neutrons, which affects their mass.
How do ions form?

+
Ions form when atoms gain or lose electrons to achieve a more stable electron configuration.
Why are nuclear forces crucial for atom stability?

+
Nuclear forces counteract the repulsive electromagnetic force between protons, ensuring the nucleus remains intact and stable.


