7 Key Enzyme Worksheet Answers Unveiled

The significance of enzymes in biology education and research cannot be overstated, as these biological catalysts are essential for a myriad of life-sustaining reactions. Educators and students often rely on enzyme worksheets to deepen their understanding of these proteins. This post will unveil answers to seven key questions often found in enzyme worksheets, providing insights into the nature, function, and mechanisms of enzymes. Each section will delve into different aspects of enzyme behavior, ensuring a comprehensive grasp of this fundamental topic in biochemistry.
What is an Enzyme?

An enzyme is a type of protein that serves as a catalyst, accelerating chemical reactions by lowering the activation energy required for these reactions to proceed. They facilitate this by binding with the substrate, the molecule upon which they act, to form an enzyme-substrate complex. Here are key points about enzymes:
- Speed up reactions: Without changing the net reaction themselves, enzymes increase the rate of reactions.
- Highly Specific: Each enzyme typically binds to only one or a few substrates with high specificity.
- Reusable: After catalyzing a reaction, the enzyme remains unchanged and can act on additional substrates.

How Do Enzymes Work?

Enzyme catalysis involves several critical steps:
- Enzyme-Substrate Binding: The enzyme binds to the substrate at an active site, forming an enzyme-substrate complex. The shape of this site is complementary to that of the substrate.
- Catalytic Mechanism: This step involves the actual catalysis, where the enzyme facilitates the conversion of the substrate to product, often through stabilizing transition states or strain.
- Product Release: The product is released from the enzyme, leaving it free to bind to another substrate molecule.
What Affects Enzyme Activity?

Several factors influence enzyme activity:
- Temperature: Increasing temperature can increase activity up to an optimum point, after which denaturation occurs.
- pH: Enzymes have an optimal pH range where their activity is maximized.
- Substrate Concentration: At low concentrations, activity increases with more substrate; saturation occurs when all enzyme active sites are occupied.
- Enzyme Concentration: More enzyme means more reaction rate, but this is limited by substrate availability.
Enzyme Inhibitors: What Are They?

Enzyme inhibitors are substances that reduce the activity of enzymes by:
- Competitive Inhibition: Inhibitors compete with the substrate for the active site.
- Non-competitive Inhibition: Inhibitors bind to a site other than the active site, changing the enzyme’s structure or function.
- Uncompetitive Inhibition: Inhibitors only bind to the enzyme-substrate complex, further slowing or stopping the reaction.
⚠️ Note: Enzyme inhibitors are crucial in medical treatments and biological research for controlling metabolic pathways.
Enzyme Kinetics: Understanding Michaelis-Menten Equation

The Michaelis-Menten equation describes the relationship between reaction velocity and substrate concentration:
- Km (Michaelis constant): Measures the affinity of the enzyme for its substrate; lower Km means higher affinity.
- Vmax (maximum velocity): The maximum rate the enzyme can achieve with an unlimited supply of substrate.
| Term | Definition |
|---|---|
| Km | Michaelis-Menten constant, indicating substrate affinity |
| Vmax | Maximal rate of enzymatic reaction at substrate saturation |

Importance of Coenzymes and Cofactors

Coenzymes and cofactors are essential for enzyme function:
- Coenzymes: Organic compounds that assist in catalysis by carrying chemical groups or electrons between enzymes.
- Cofactors: Inorganic substances or metal ions required for enzyme activity.
What are Enzyme Deficiencies?

Enzyme deficiencies can lead to various metabolic disorders:
- Examples include phenylketonuria (PKU) due to a lack of phenylalanine hydroxylase or glucose-6-phosphate dehydrogenase deficiency.
- Treatment might involve diet management, supplements, or gene therapy to restore enzyme functionality.
In sum, enzymes are the linchpins of biological systems, controlling reactions with precision and efficiency. Through their study, we've explored their structures, functions, and the impacts of various inhibitors and deficiencies. Their activity is modulated by conditions like temperature and pH, requiring careful consideration in experimental settings. The knowledge gained from enzyme worksheets is not only fundamental for academic purposes but also for understanding health, disease, and the future of biotechnology.
What is the role of pH in enzyme activity?

+
pH affects enzyme activity by altering the ionization state of amino acids in the enzyme’s active site, which in turn changes the enzyme’s shape and its ability to bind with the substrate effectively.
Why do enzymes show specificity?
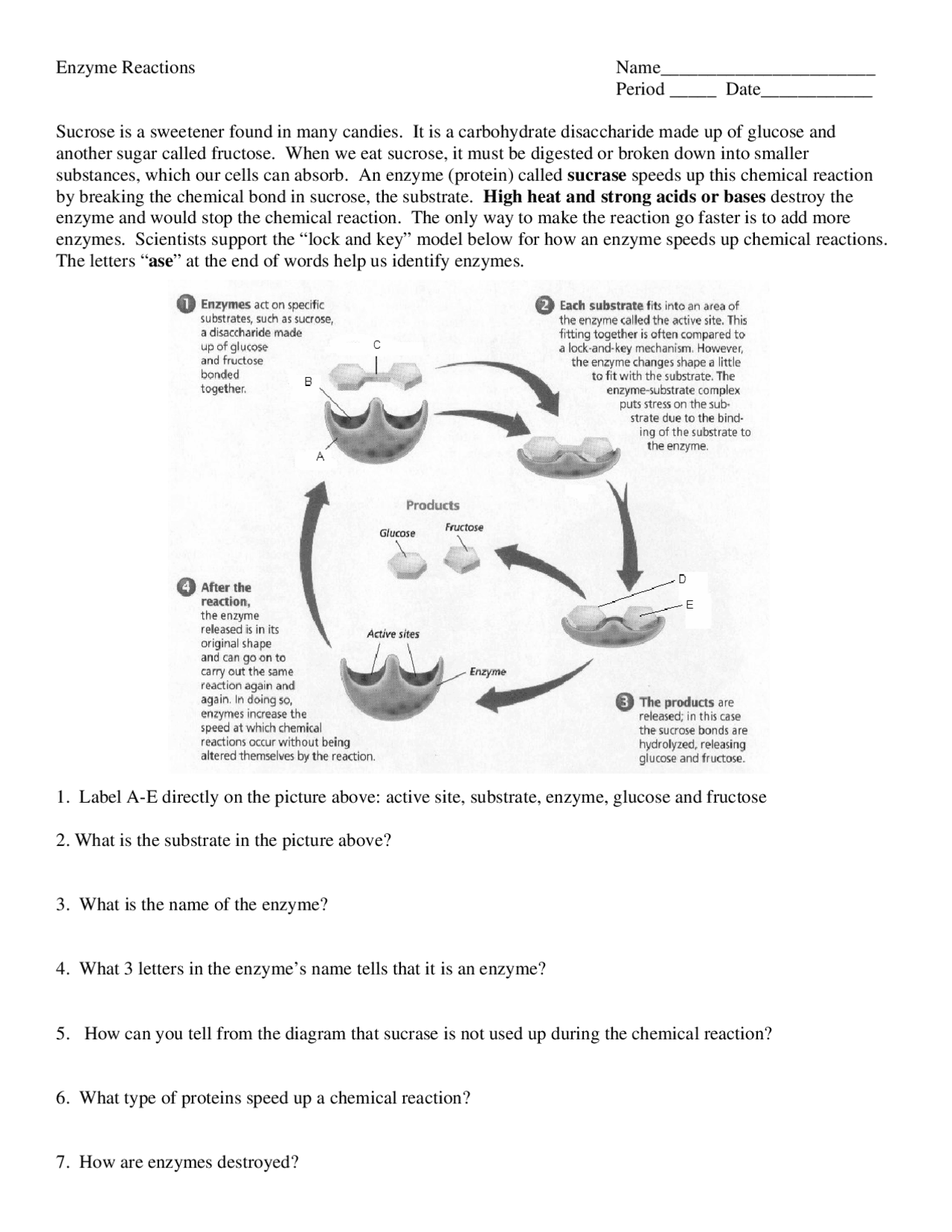
+
Enzymes exhibit specificity due to the unique fit of the substrate into the enzyme’s active site, shaped by the enzyme’s three-dimensional structure which complements the substrate’s molecular configuration.
How can enzyme deficiencies be treated?

+
Treatment for enzyme deficiencies includes dietary modifications to bypass the enzyme’s function, supplementation with the deficient enzyme, or emerging treatments like gene therapy to correct or replace the faulty enzyme-producing gene.