Writing And Naming Binary Compounds Worksheet

Introduction to Naming Binary Compounds

Welcome to our comprehensive guide on writing and naming binary compounds! Whether you're a student grappling with chemistry assignments, or simply someone curious about the language of chemistry, this guide will walk you through the systematic process of naming compounds consisting of two different elements. Understanding how to name binary compounds is fundamental in the study of chemistry, as it allows scientists to communicate clearly and concisely about chemical substances.
Understanding Binary Compounds

A binary compound is a chemical compound consisting of exactly two different elements. These elements can be metals with nonmetals or two nonmetals. Here's a brief overview:
- Metal with Nonmetal: These often form ionic compounds, where one element transfers electrons to another to achieve a full outer shell.
- Nonmetal with Nonmetal: These generally form covalent compounds, where atoms share electrons.
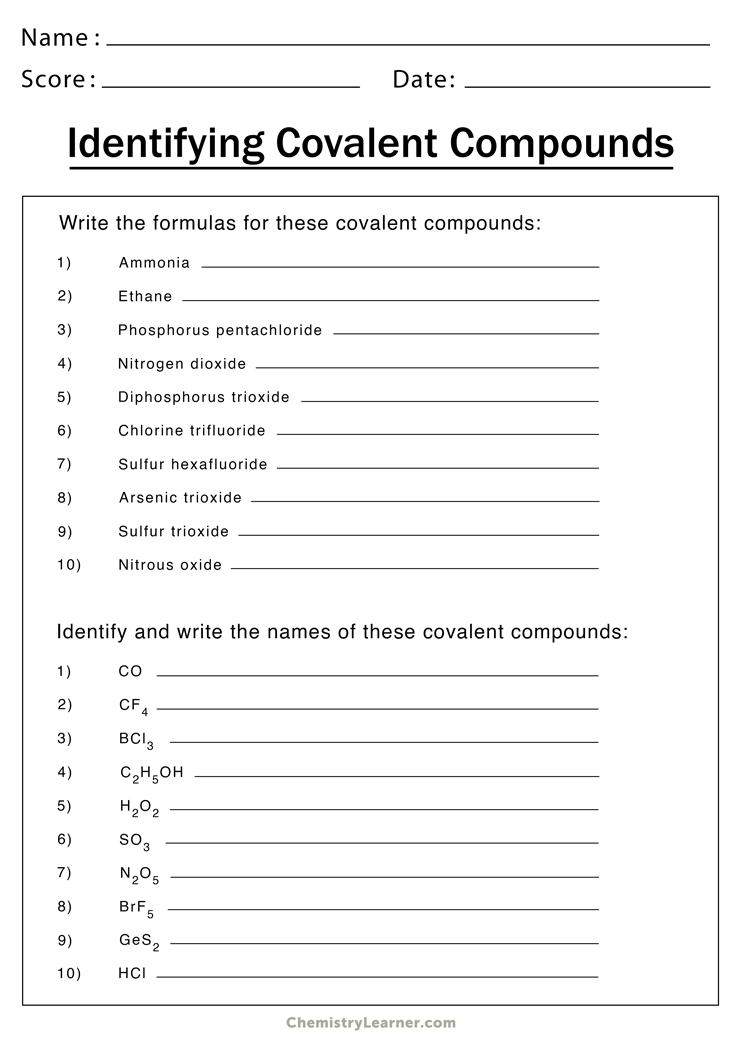
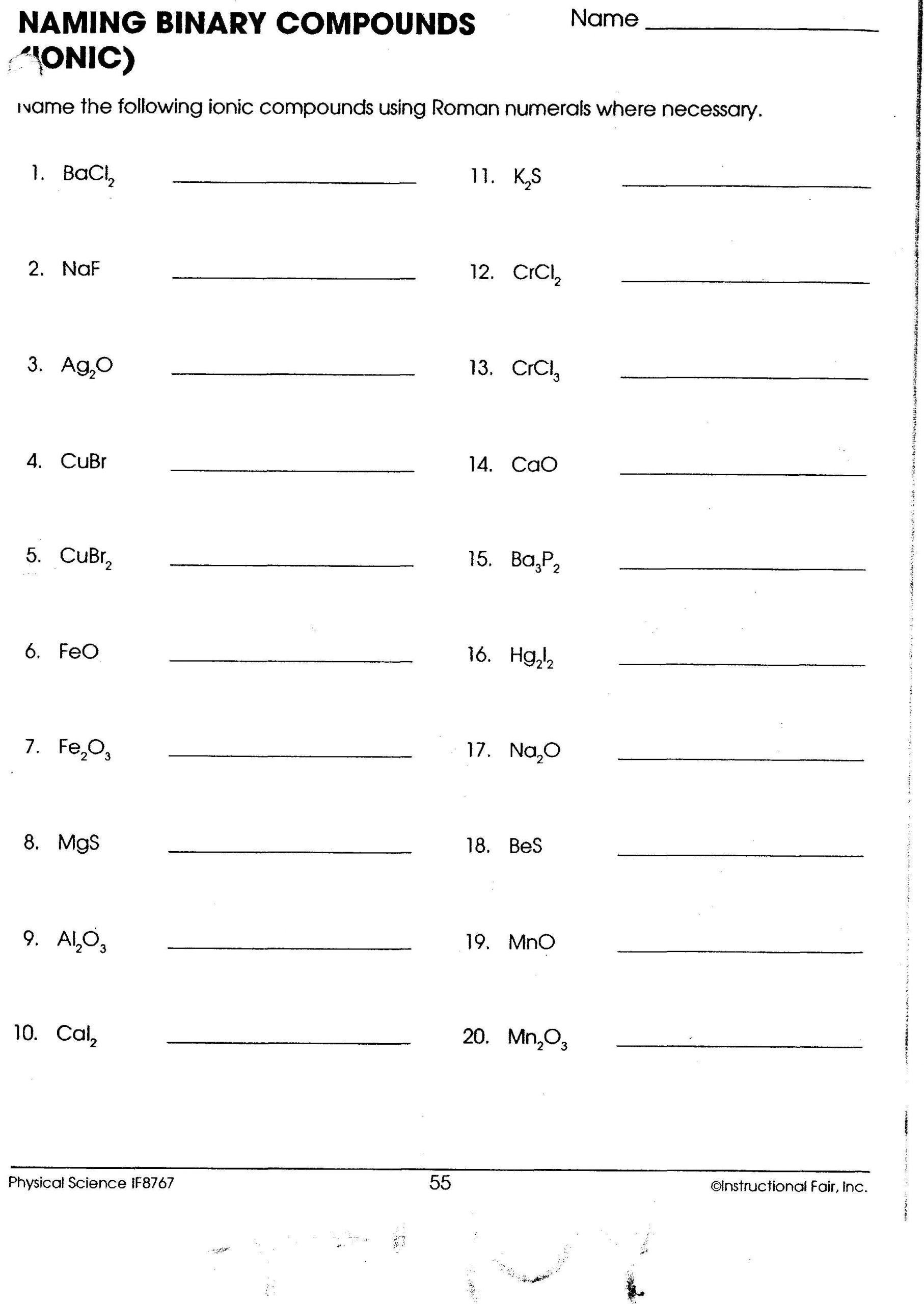
Basic Rules for Naming Ionic Compounds

Naming ionic compounds involves identifying the metal and nonmetal components and following these steps:
1. Name the Metal First

Write the name of the metal in the compound first:
- If the metal is in Group 1 or 2 of the periodic table, it has only one possible charge, so you use its elemental name.
- For metals in transition groups or post-transition metals that can have variable charges (e.g., iron, copper), you must specify the charge using Roman numerals in parentheses (this is known as the Stock System).
2. Name the Nonmetal with an -ide Ending

The nonmetal part of the compound is named by taking the root of its element name and adding the suffix -ide:
- Chlorine becomes chloride.
- Fluorine becomes fluoride.
💡 Note: Remember that when the anion has a variable charge (like in the case of transition metals), you might need to use Roman numerals to indicate the charge on the metal ion.
3. Example of Naming Ionic Compounds
Here’s how you might name NaCl (table salt):
- Sodium - because sodium is from group 1, it has only one charge.
- Chloride - chlorine as a nonmetal gets the -ide suffix.
The compound is named sodium chloride.
| Compound | Name |
|---|---|
| NaCl | Sodium chloride |
| FeO | Iron(II) oxide |
| CuBr2 | Copper(II) bromide |

Basic Rules for Naming Covalent Compounds
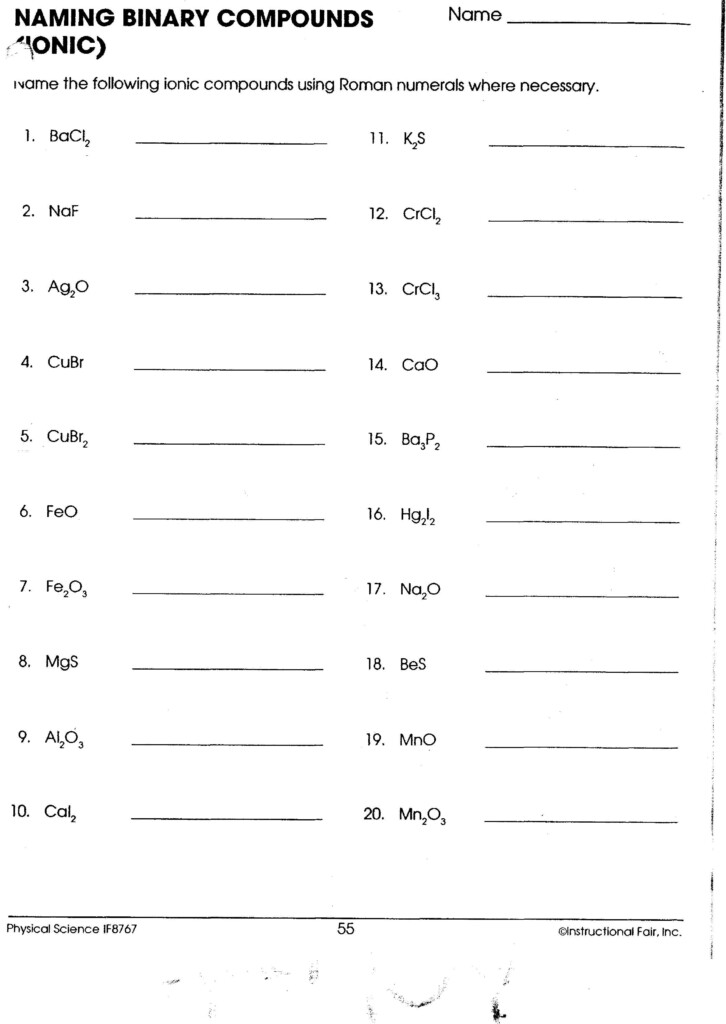
Naming covalent compounds uses Greek prefixes to indicate the number of atoms of each element:
1. Use Greek Prefixes

- mono- (1), di- (2), tri- (3), tetra- (4), penta- (5), hexa- (6), hepta- (7), octa- (8), nona- (9), deca- (10).
2. Write the Less Electronegative Element First

The less electronegative (or more electropositive) element is written first:
- CO: Carbon monoxide.
- P2O5: Diphosphorus pentoxide.
3. Use Prefixes to Specify the Number of Atoms

- If there's only one atom of the first element, the prefix "mono" is usually omitted except when it is necessary for clarity, like in "monoxide" for CO.
- End the name of the last element with -ide.
4. Example of Naming Covalent Compounds

Here’s how you might name N2O:
- Dinitrogen - because there are two nitrogen atoms.
- Monoxide - because there's only one oxygen atom, but you include "mono" here for clarity.
The compound is named dinitrogen monoxide.
In summary, this guide has explored the key principles behind naming binary compounds. Whether dealing with ionic or covalent compounds, the process involves straightforward rules that can be mastered with practice. Understanding these naming conventions helps in:
- Communication: Scientists worldwide can discuss compounds without confusion.
- Synthesis: Knowing names can aid in predicting reactions and compound properties.
- Education: It’s crucial for students to grasp these basics for further studies in chemistry.
By following these steps, you’ll be well-equipped to handle various chemical naming scenarios, from simple salts like NaCl to more complex molecules like CO2 or FeCl3.
What is the difference between ionic and covalent compounds?

+
Ionic compounds are formed when one element transfers electrons to another, creating ions that are held together by electrostatic forces. Covalent compounds, on the other hand, involve the sharing of electrons between atoms.
How do you know if a metal needs a Roman numeral in its name?

+
If the metal can have more than one common ionic charge (like transition metals), it requires a Roman numeral to denote its oxidation state in the compound.
Why don’t covalent compounds use Roman numerals?
+
Covalent compounds do not typically involve ion formation with multiple charge states, so they use prefixes to indicate the number of atoms instead.
Can you name a compound without knowing its formula?
+It’s generally not possible to name a compound accurately without its formula, as the formula indicates the type and number of atoms in the compound, which is critical for naming.