5 Tips to Master Periodic Trends Worksheet Easily

Understanding periodic trends is crucial for any student delving into the world of chemistry. These trends allow chemists to predict properties like atomic size, ionization energy, electron affinity, and electronegativity based on an element's position in the periodic table. Mastering periodic trends worksheets is not just about memorizing but understanding why these trends exist. Here are five tips to help you navigate these worksheets with ease:
Understand the Basics of the Periodic Table

Before diving into trends, you need a solid foundation in the periodic table:
- Groups and Periods: Know that elements in the same group share similar properties because they have the same number of valence electrons.
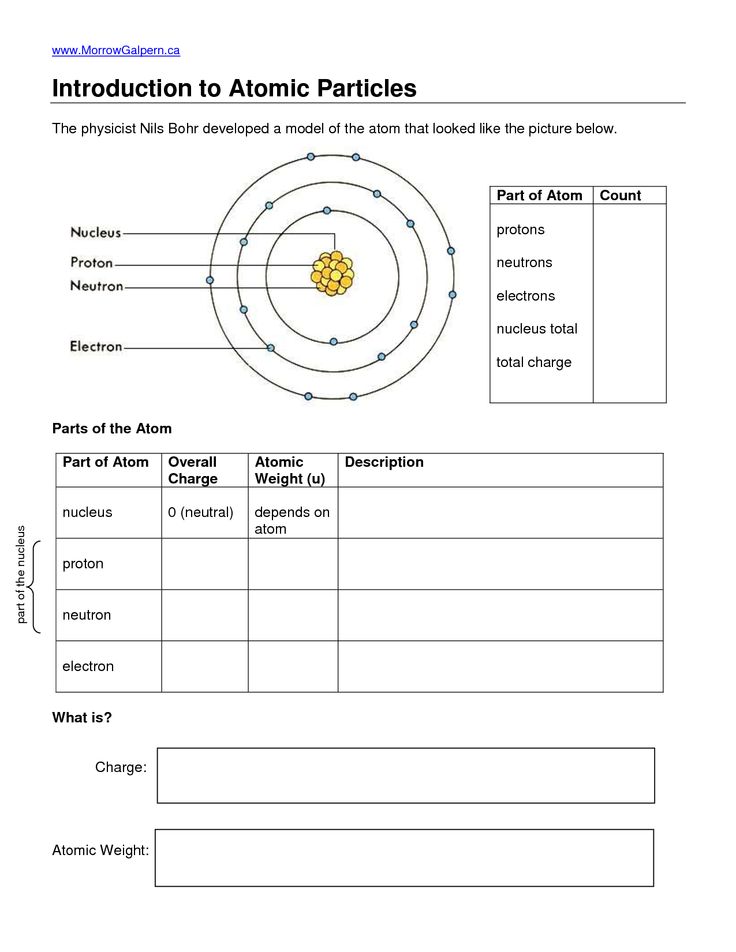
- Atomic Number: Each element’s atomic number reflects the number of protons in the nucleus, which is pivotal in understanding trends.
- Shells and Subshells: Be familiar with how electrons are arranged in shells and subshells, as this influences many periodic properties.
🔍 Note: The periodic table isn't just a grid; it's a map that reveals the nature of elements through their positions.
Identify and Memorize Key Trends

Here are the main periodic trends you should focus on:
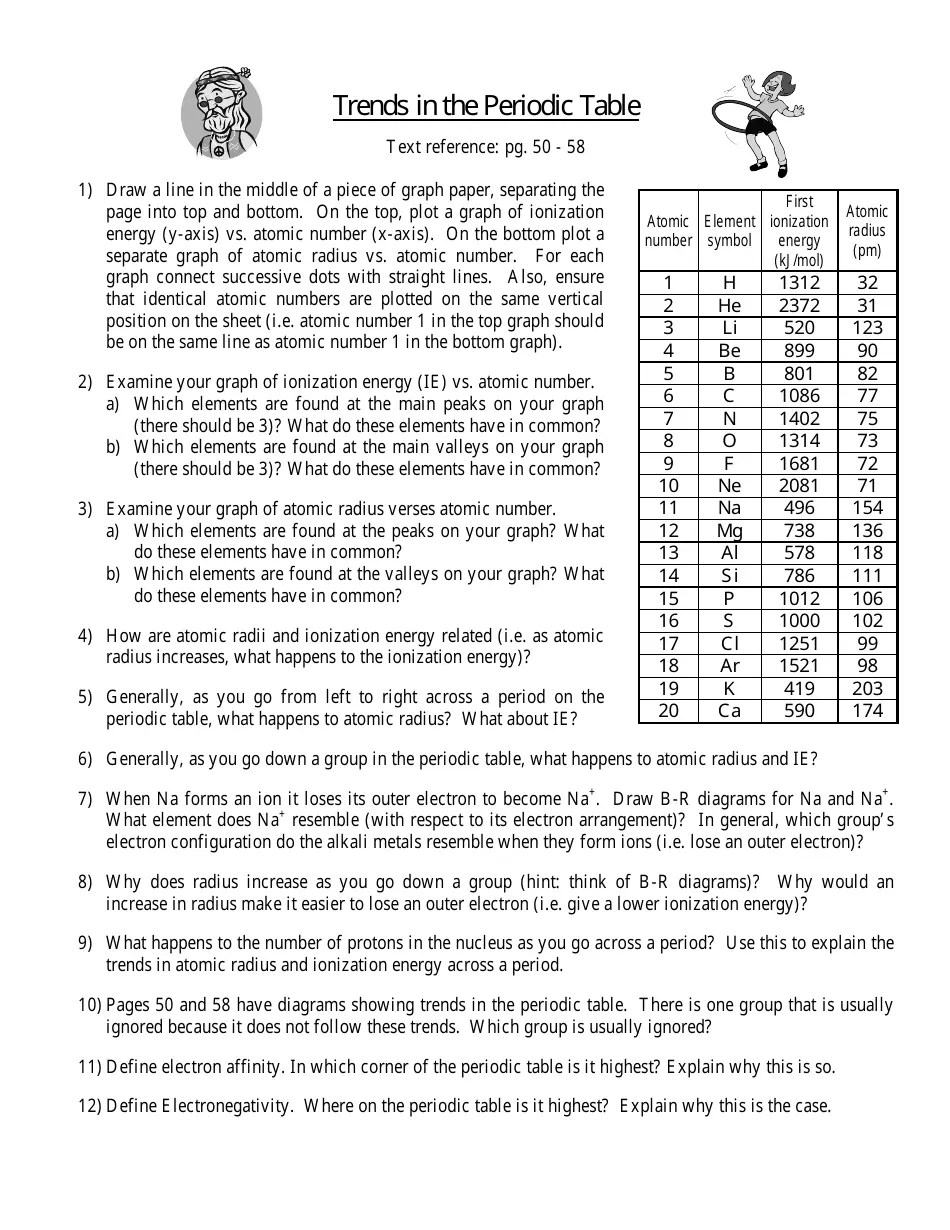
- Atomic Radius: Increases down a group (due to additional energy levels) and decreases across a period (due to increased nuclear charge).
- Ionization Energy: Increases across a period and decreases down a group because as you move down, the electrons are further from the nucleus.
- Electron Affinity: Generally increases across a period, with some exceptions due to electron configuration.
- Electronegativity: Increases both from left to right across a period and from bottom to top within a group.
- Metallic Character: Increases down a group and decreases across a period.
| Property | Trend Across Periods (L to R) | Trend Down Groups |
|---|---|---|
| Atomic Radius | Decreases | Increases |
| Ionization Energy | Increases | Decreases |
| Electron Affinity | Increases | Decreases |
| Electronegativity | Increases | Decreases |
| Metallic Character | Decreases | Increases |

Relate Trends to Electron Configurations

Electron configuration plays a significant role in periodic trends:
- Elements within the same period fill the same energy level, which directly affects ionization energy and electron affinity.
- The shape of the electron cloud, or orbital, can influence how electrons interact with each other, affecting the trends.
- The stability of electron configurations (like noble gas configurations) can lead to exceptions in trends like electron affinity.
Use Mnemonics and Visual Aids

Visual aids and mnemonics can help you remember and understand periodic trends:
- Arrow Diagrams: Draw arrows representing trends moving across periods or down groups.
- Color Coding: Use different colors for different trends on a periodic table printout.
- Mnemonic Devices: Create phrases or acronyms to remember trends, like ‘Rabbits In My Backyard Eat Carrots’ for Atomic Radius, Ionization Energy, Electron Affinity, Electronegativity, and Metallic character.
Practice With Varied Examples

Consistent practice with different elements helps solidify your understanding:
- Start with elements within the same group or period to see the direct trends.
- Challenge yourself by comparing elements that are further apart.
- Use online resources or textbooks to find practice worksheets or quizzes.
- Participate in group discussions or study groups to discuss and clarify doubts.
📚 Note: The more varied your practice, the better you'll grasp the nuances of periodic trends.
The art of mastering periodic trends worksheets lies not just in memorizing facts but in deeply understanding why elements behave as they do based on their positions in the periodic table. By focusing on these tips, you can navigate the complexities of periodic trends with confidence:
- Understanding the Basics: Familiarity with the periodic table's structure is your first step towards mastery.
- Key Trends: Know and apply the main trends to predict element properties effectively.
- Electron Configuration: Link trends back to the arrangement of electrons, which provides a logical explanation for the observed patterns.
- Mnemonics and Visuals: These tools can simplify the learning process, making it more intuitive.
- Practice: Constant practice with a variety of elements ensures you can apply trends knowledge across different scenarios.
Remember, chemistry is about understanding patterns and predicting behaviors. By grasping these periodic trends, you're equipping yourself with the tools to excel in chemistry, making it easier to tackle more advanced topics in the future. This foundational knowledge will serve as a springboard for further learning, ensuring that your journey through the sciences is both efficient and insightful.
Why do periodic trends matter in chemistry?

+
Periodic trends provide insights into chemical behavior, helping predict how elements will react with others, their physical properties, and the bonding they form. Understanding these trends is fundamental to predicting and understanding chemical reactions.
How can I remember all the periodic trends?

+
Using mnemonics, color-coding, and regular practice can help. Relating trends to electron configurations also provides a deeper understanding, making the trends easier to remember.
What are common mistakes students make with periodic trends?
+
Common mistakes include mixing up trends across periods and down groups, neglecting the influence of electron configuration, and not considering exceptions due to electron stability. Also, focusing too much on memorization without understanding the ‘why’ behind trends can lead to confusion.