7 Key Periodic Trends Every Chemistry Student Should Know
Understanding the periodic table is a cornerstone for students studying chemistry, as it provides a wealth of information about elements and their properties. Among the myriad of patterns, seven key periodic trends stand out due to their fundamental importance in understanding how elements behave and interact:
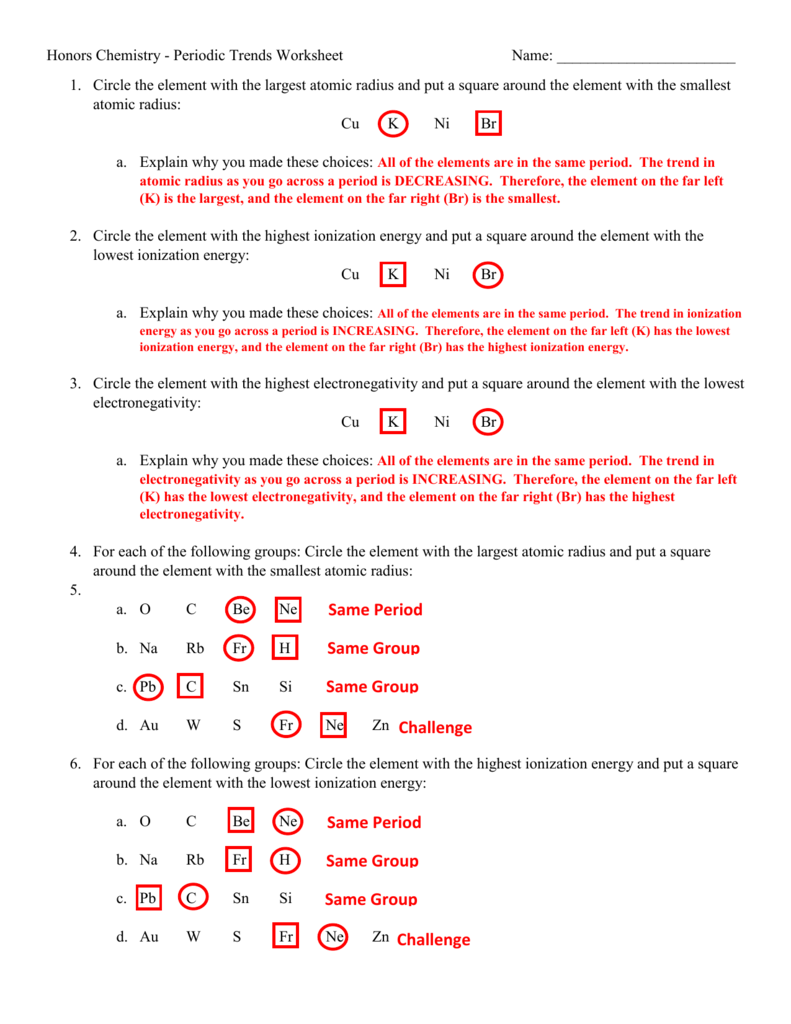
1. Atomic Radius


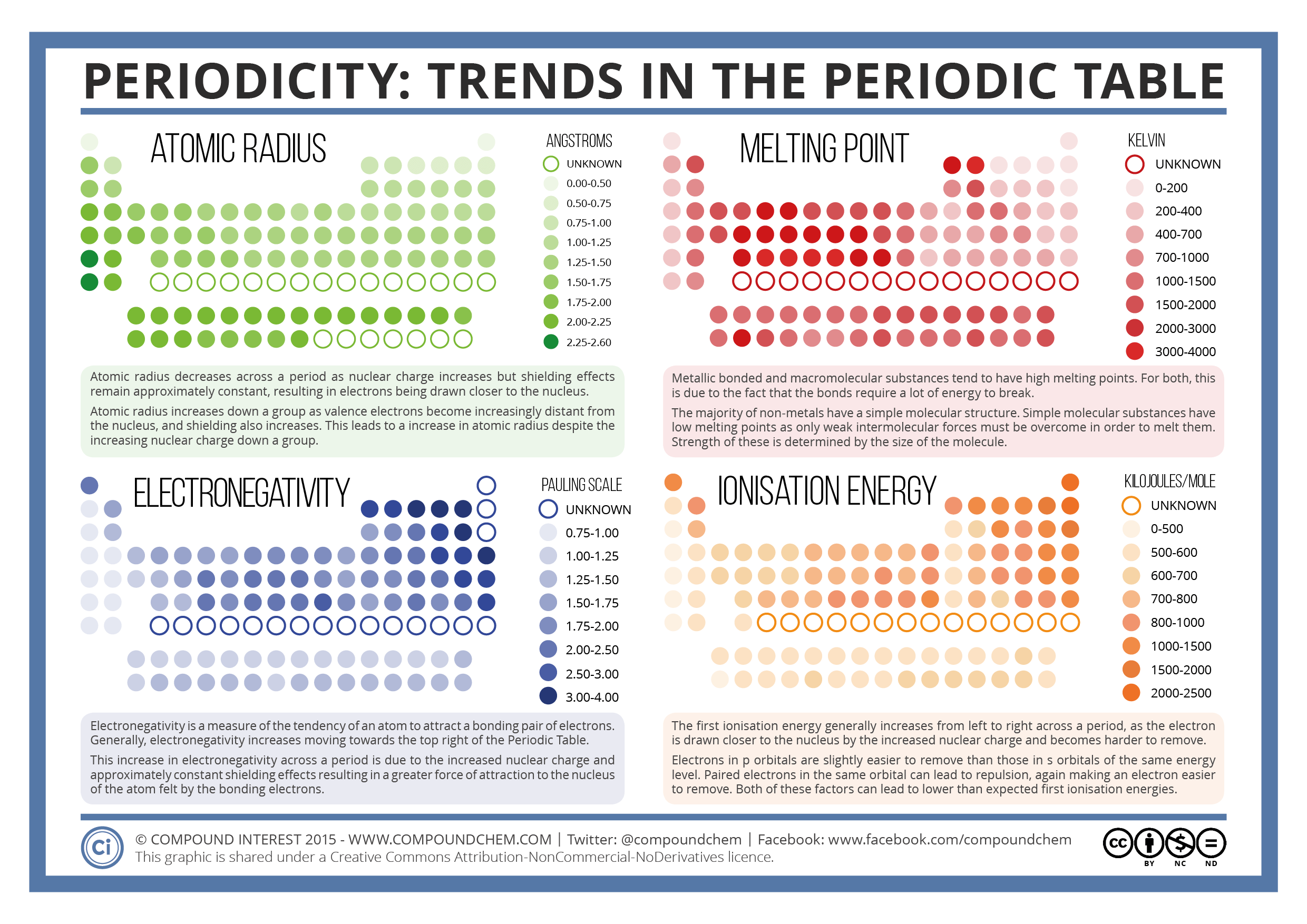
Atomic radius refers to the size of an atom, which decreases across a period from left to right and increases down a group:
- Across a Period: As the effective nuclear charge increases due to the addition of protons, electrons are pulled closer to the nucleus, reducing the atomic radius.
- Down a Group: Electrons are added in higher energy levels, further from the nucleus, causing an increase in atomic size.
2. Ionic Radius

Upon losing or gaining electrons, atoms become ions. Here's how their radii change:
- Cations: Positive ions have a smaller radius than the parent atoms due to the loss of an entire shell of electrons.
- Anions: Negative ions have a larger radius because they gain electrons, resulting in increased repulsion between electrons.
3. Ionization Energy


This is the energy required to remove an electron from a gaseous atom or ion. The trend is as follows:
- Across a Period: Ionization energy increases due to increasing nuclear charge, pulling electrons closer to the nucleus, making them harder to remove.
- Down a Group: Ionization energy decreases as the electrons are farther from the nucleus and feel less attraction.
4. Electron Affinity

Electron affinity measures the energy change when an atom in its gaseous state gains an electron:
- Across a Period: Electron affinity generally increases as the nuclear attraction for additional electrons strengthens.
- Down a Group: Electron affinity decreases due to the larger atomic radii, which reduces the effective attraction for an extra electron.
5. Electronegativity

Electronegativity is the ability of an atom in a molecule to attract shared electrons towards itself:
- Across a Period: Electronegativity increases due to the increase in nuclear charge.
- Down a Group: It decreases as the size of the atom increases, and the nucleus has less control over shared electrons.
| Element | Electronegativity (Pauling Scale) |
|---|---|
| Fluorine (F) | 3.98 |
| Oxygen (O) | 3.44 |
| Chlorine (Cl) | 3.16 |
| Nitrogen (N) | 3.04 |
| Sodium (Na) | 0.93 |

6. Metallic and Non-metallic Character

These characteristics reflect how elements behave chemically:
- Metallic Character: Increases down a group due to easier loss of electrons.
- Non-metallic Character: Increases across a period due to the stronger pull of the nucleus on electrons, making them more likely to gain electrons.
7. Reactivity
Reactivity is how vigorously an element reacts with other substances:
- Metals: Reactivity increases down a group because larger atoms lose their valence electrons more easily.
- Non-metals: Reactivity decreases down a group as the electron shell is further from the nucleus, and atoms are less likely to gain electrons.
💡 Note: Understanding these periodic trends not only helps in predicting chemical behavior but also forms the basis for understanding chemical reactions, bonding, and material properties.
In summary, periodic trends provide a systematic way to understand the chemical properties of elements. They help chemists predict how elements will react, form compounds, and behave in various chemical and physical environments. By mastering these trends, students and scientists can better comprehend the essence of chemical science, from simple ion formation to complex reaction mechanisms.
What causes the atomic radius to change across the periodic table?

+
The atomic radius decreases across a period due to increasing effective nuclear charge, which pulls electrons closer to the nucleus. Conversely, it increases down a group because new electron shells are added further from the nucleus, increasing the size of the atom.
How do you predict the ionization energy of an element?

+
Ionization energy increases across a period because electrons are closer to the nucleus, making them harder to remove. It decreases down a group due to the increasing distance between the outermost electrons and the nucleus, thus requiring less energy to remove them.
Why do metals become more reactive down a group?

+
As you move down a group, metals tend to lose their valence electrons more readily because these electrons are further from the nucleus and are less tightly bound, making it easier for metals to form positive ions, thus increasing reactivity.



