5 Key Ways to Organize the Periodic Table

Understanding and organizing the periodic table is fundamental for chemistry students, researchers, and educators. The periodic table isn't just a chart of elements; it's a blueprint that helps us predict how elements might react with one another and how they might behave under certain conditions. Here are five key ways to organize the periodic table for enhanced comprehension and utility:
1. By Atomic Number
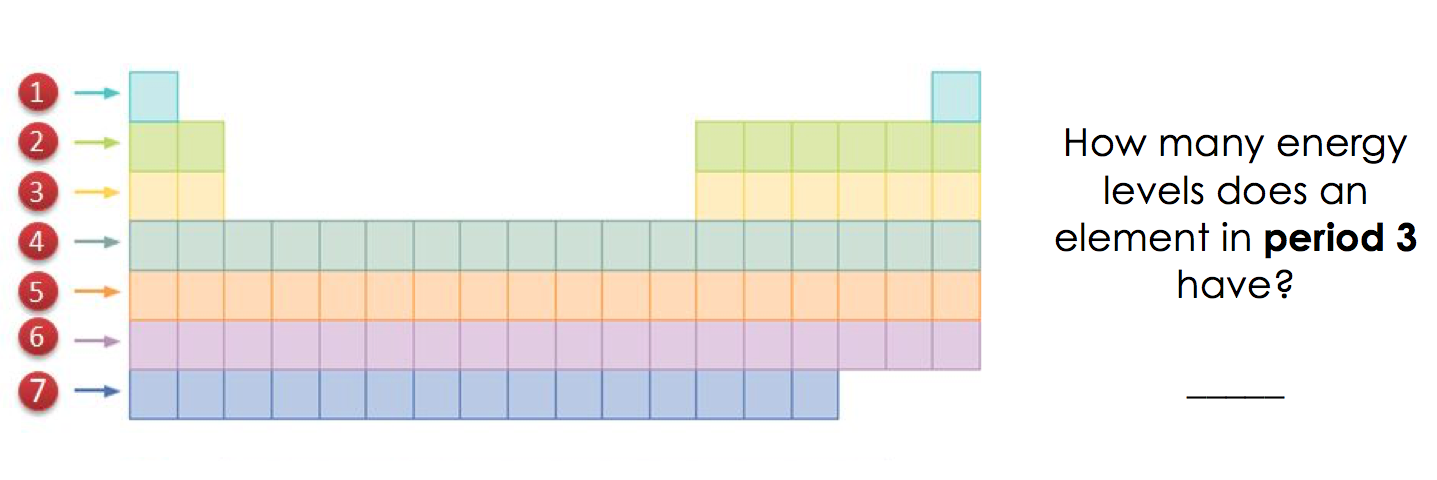
The most common and perhaps the most logical way to organize the periodic table is by the atomic number. This is essentially the number of protons in an element’s nucleus, which defines the element’s identity. Here’s how you can effectively use this method:
- Order Elements: Arrange the elements in ascending order of atomic number. Hydrogen, with an atomic number of 1, sits at the top-left corner, while higher atomic numbers fill up the rows and columns, ending with the heaviest elements like Oganesson with an atomic number of 118.
- Periodic Trends: Recognizing patterns like the increase in atomic radius down a group or ionization energy across a period helps in understanding element properties.
📌 Note: When arranging by atomic number, elements are grouped in a manner that aligns with the Aufbau principle, which states that electrons fill orbitals starting from the lowest energy level to the highest.
2. By Electron Configuration
Electron configuration reveals the distribution of electrons in an atom’s energy levels, which directly influences chemical properties:
- Group Elements: Elements in the same group have similar electron configurations, which leads to shared chemical behavior. For example, all elements in Group 1 have an electron configuration ending in s1.
- Identify Shells and Subshells: You can categorize elements based on whether their outermost electrons are in the s, p, d, or f subshells, providing insight into element reactivity and compound formation.
📌 Note: The lanthanides and actinides, often shown separately, can be better understood by considering their electron configurations, which involve f-orbital filling.
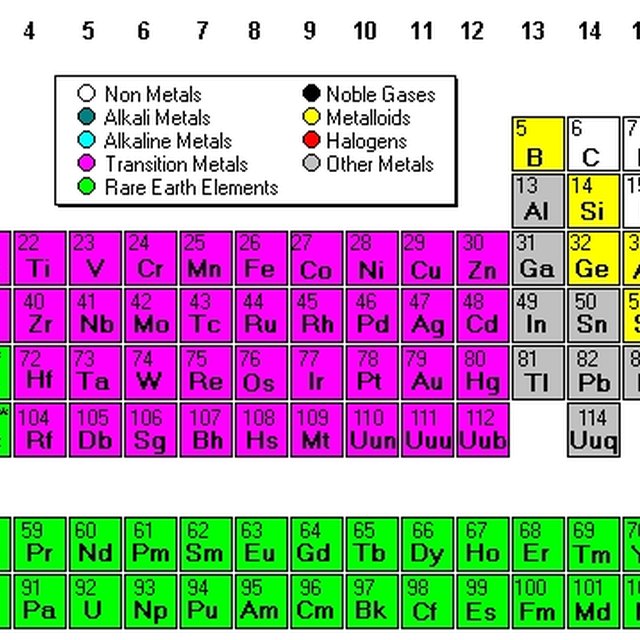
3. By Chemical Properties

Organizing by chemical behavior allows for easy prediction of reactions:
- Periodic Groups: Elements with similar chemical properties are grouped together. Alkali metals, alkaline earth metals, transition metals, nonmetals, metalloids, halogens, and noble gases are all placed in their respective columns.
- Reactivity Trends: Understanding where an element sits relative to its reactivity can aid in chemical analysis and synthesis.
| Group | Chemical Property |
|---|---|
| 1 | Highly reactive metals |
| 18 | Very unreactive (Noble Gases) |
| 7 | Reactive non-metals (Halogens) |

4. By Physical Properties

The periodic table can also be organized by physical attributes:
- Melting and Boiling Points: Trends in these properties can be seen vertically (groups) or horizontally (periods). For instance, melting points generally increase from left to right across periods and decrease down groups in the periodic table.
- Density: Organizing by density can provide insight into element utility, especially for metals used in engineering and manufacturing.
5. By Trends and Patterns

Exploring periodic trends gives us a deeper understanding of element behavior:
- Ionization Energy: Organizing elements by their ionization energies helps in understanding how readily elements lose electrons.
- Electronegativity: Elements can be arranged to highlight their tendency to attract shared electrons, which affects bond formation and reactivity.
- Atomic Radius: Organizing by atomic radius allows for an intuitive grasp of element sizes and how they interact in compounds.
📌 Note: While these trends are generally true, there are exceptions. Recognizing these exceptions can provide even deeper insights into atomic structure and chemistry.
Summing up, organizing the periodic table in different ways provides multiple lenses through which we can understand the vast world of chemistry. Each method of organization not only highlights different aspects of the elements but also aids in learning, teaching, and applying chemistry in real-world scenarios. From atomic number and electron configuration to chemical and physical properties, and overarching trends, the periodic table remains a cornerstone of scientific knowledge, offering endless pathways to discovery and innovation.
Why is the atomic number important for organizing the periodic table?

+
The atomic number defines an element uniquely by the number of protons it contains. This number also determines the element’s electron configuration and hence its chemical behavior, making it a fundamental criterion for organizing elements in the periodic table.
What are the differences between metals and non-metals on the periodic table?
+
Metals are typically found on the left side and center of the periodic table. They tend to be lustrous, good conductors of heat and electricity, and lose electrons during chemical reactions. Non-metals, situated on the right side, are generally poor conductors, and they tend to gain or share electrons when forming compounds.
Can electron configuration influence an element’s position in the periodic table?

+
Yes, electron configuration directly influences where an element is placed in the periodic table. Elements with similar outermost electron configurations are grouped together, as they exhibit similar chemical properties due to the arrangement of their electrons.