Understanding Mole Concept with Avogadro's Number Worksheet

One of the fundamental concepts in chemistry is understanding the mole and its relationship with Avogadro's number. These concepts form the backbone of quantitative chemical analysis, stoichiometry, and various other calculations in the chemistry laboratory. In this detailed exploration, we will delve into how Avogadro's number and the mole concept work in harmony, using a worksheet to guide our understanding.
What is a Mole?

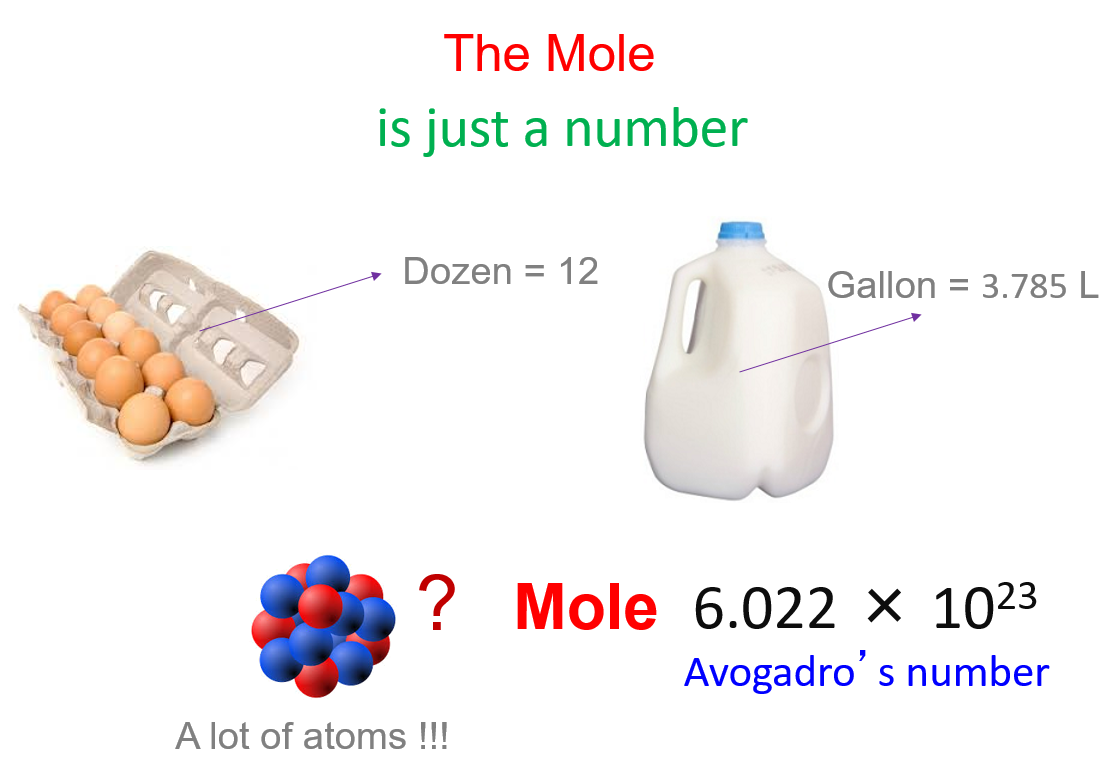
A mole (abbreviated as "mol") is the SI unit for measuring the amount of a substance. It's a counting unit much like "a dozen" or "a gross." But while a dozen refers to exactly 12 items, a mole refers to a much larger number: 6.022 x 1023, known as Avogadro's number. This number is crucial because:
- It represents the number of atoms, ions, or molecules in a gram-molecular weight or molar mass of a substance.
- It allows for the conversion between the mass of a substance and the number of particles.
Avogadro's Number

Named after the Italian scientist Amedeo Avogadro, this number (6.022 x 1023) is the key to understanding how many particles are in one mole of a substance. Here's why it's significant:
- It quantifies the amount of a substance in a way that is universally comparable, regardless of the size or complexity of the molecule.
- It enables us to relate the microscopic world of atoms and molecules to the macroscopic world of measurements in grams.
🔍 Note: Avogadro's number is an experimentally determined value, with a very small margin of uncertainty.
The Mole Concept in Practice

Let's see how the mole and Avogadro's number interact in a practical worksheet setting:
Mole to Mass Calculation

To convert moles to grams, you use the molar mass of the substance. Here's a simple table to illustrate:
| Element | Molecular Mass (amu) | Moles | Gram Mass (g) |
|---|---|---|---|
| Carbon (C) | 12.01 | 1 | 12.01 |
| Hydrogen (H2) | 2.02 | 2 | 4.04 |
| Water (H2O) | 18.02 | 1 | 18.02 |

From this table, you can see how the moles of a substance relate to its molecular mass to give the gram mass:
- To find the mass of 1 mole of Carbon (C), you multiply the molecular mass by 1, resulting in 12.01 grams.
- For 2 moles of Hydrogen (H2), the calculation is 2 * 2.02 = 4.04 grams.
- Water (H2O) has one oxygen atom (16.00 amu) and two hydrogen atoms (2.02 amu), totaling 18.02 amu per molecule, so 1 mole of H2O weighs 18.02 grams.
Mass to Mole Calculation
The reverse calculation also applies:
- To find the number of moles given the mass, you divide the mass (in grams) by the molar mass.
- So, if you have 24 grams of Carbon (C), you have (24 g / 12.01 g/mol) = 1.9983 moles, approximately 2 moles.
Avogadro's Number Worksheets

Worksheets focusing on Avogadro's number and the mole concept typically involve:
- Conversions between moles and mass.
- Calculations involving Avogadro's number to determine the number of atoms or molecules in a sample.
- Practical problem solving using the ideal gas law where Avogadro's law (n = V/RT) plays a crucial role.
Application in Stoichiometry

In stoichiometry, we often use the mole concept to balance chemical equations and to calculate reaction yields:
- Balancing equations involves understanding how many moles of reactants combine to form products.
- Theoretical yield calculations use moles to predict the mass of products that can be formed from a given mass of reactants.
Mole Concept and Avogadro's Number: Recap and Beyond

The understanding of the mole concept and Avogadro's number is not just confined to the classroom but extends to various fields:
- Pharmaceutical chemistry for drug dosage calculation.
- Environmental science for pollutant quantification.
- Material science for analyzing composition and structure.
By mastering these concepts through Avogadro's number worksheets, students and professionals can approach chemical analysis with confidence and precision.
In summary, understanding the mole concept and Avogadro's number is pivotal in chemistry. It provides the necessary tools to:
- Quantify substances accurately.
- Perform stoichiometric calculations.
- Understand the relationships between macroscopic measurements and microscopic quantities.
What is the importance of Avogadro’s number?

+
Avogadro’s number is essential because it provides a bridge between the mass of a substance and the number of particles, enabling precise chemical calculations and understanding.
How do I calculate moles from grams?
+
To calculate moles from grams, divide the mass of the substance by its molar mass. For example, to find the number of moles in 48 grams of oxygen (O2), you would use the molar mass of O2 (32 g/mol), resulting in 48g / 32g/mol = 1.5 moles.
Why is the mole concept essential in chemistry?

+
The mole concept is fundamental for quantifying substances in terms that allow for consistent and comparable measurements, facilitating stoichiometry, reaction balancing, and experimental design.