6 Ways to Master Stoichiometry Limiting Reagent

Stoichiometry, the quantitative relationship between reactants and products in chemical reactions, can be a daunting subject for many students. One of the key concepts in stoichiometry is the limiting reagent, which determines the maximum amount of product that can be formed in a reaction. Mastering stoichiometry limiting reagent is crucial to succeed in chemistry, and here are six ways to achieve it:
1. Understand the Concept of Limiting Reagent

Before diving into calculations, it’s essential to understand the concept of limiting reagent. The limiting reagent is the reactant that is completely consumed in a reaction, thereby limiting the amount of product that can be formed. To identify the limiting reagent, you need to calculate the mole ratio of reactants and products.
2. Practice Calculating Mole Ratios

Calculating mole ratios is a crucial step in determining the limiting reagent. Practice calculating mole ratios using different types of reactions, such as combustion reactions, synthesis reactions, and decomposition reactions. You can use online calculators or worksheets to practice.
3. Use the ICE Table Method

The ICE (Initial, Change, Equilibrium) table method is a powerful tool for solving stoichiometry problems. The ICE table helps you organize your calculations and identify the limiting reagent. To use the ICE table method, follow these steps:
- List the reactants and products in the reaction.
- Calculate the initial moles of each reactant and product.
- Determine the change in moles of each reactant and product.
- Calculate the equilibrium moles of each reactant and product.
📝 Note: The ICE table method is a powerful tool for solving stoichiometry problems, but it can be time-consuming. Practice using the ICE table method to become proficient.
4. Learn to Balance Equations

Balancing equations is a critical step in stoichiometry. To balance an equation, you need to ensure that the number of atoms of each element is the same on both the reactant and product sides. Practice balancing equations using different types of reactions.
5. Use Online Resources and Worksheets
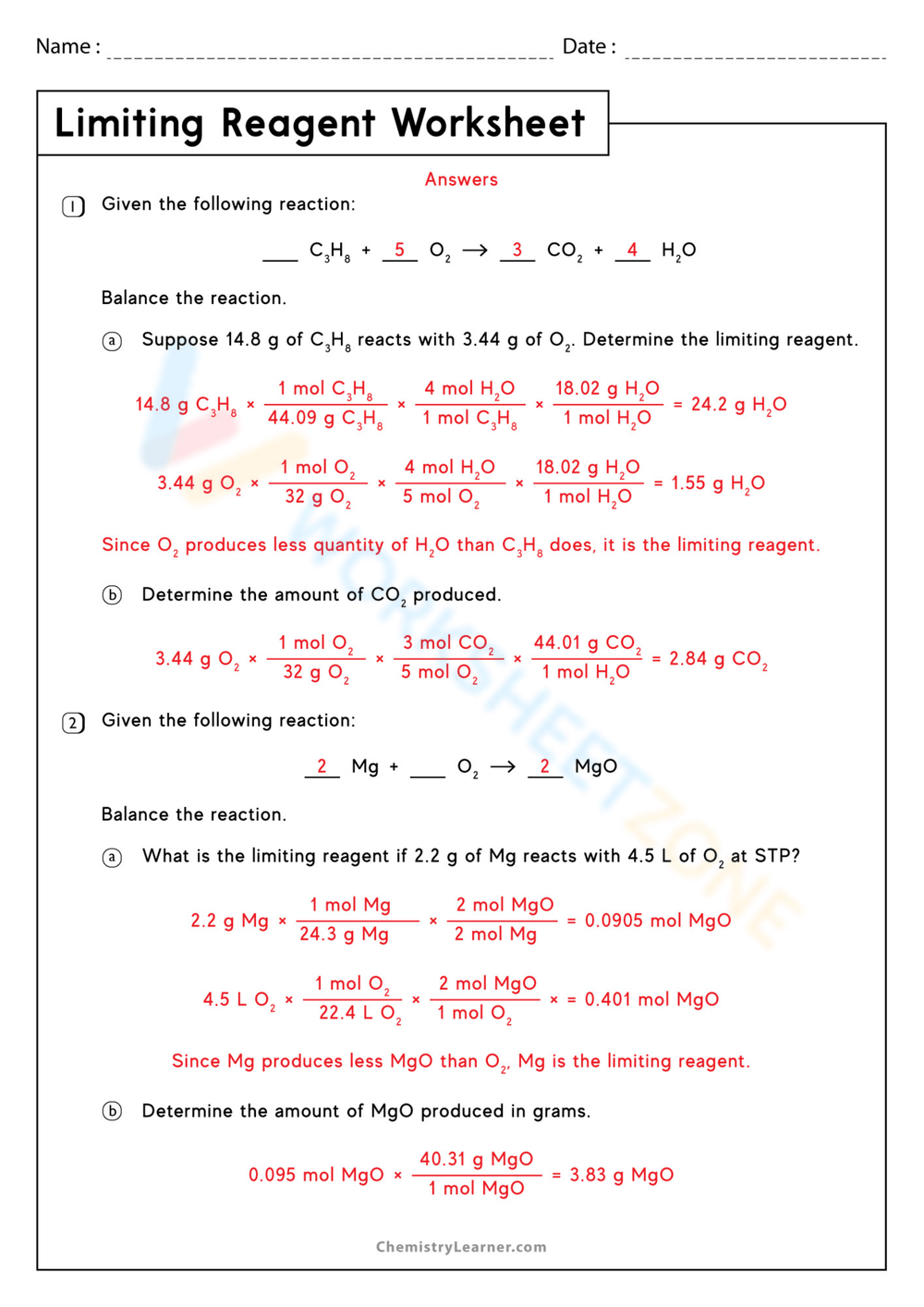
There are many online resources and worksheets available to help you practice stoichiometry limiting reagent. Websites such as Khan Academy, Crash Course, and Stoichiometry.org offer video tutorials, practice problems, and worksheets to help you master stoichiometry limiting reagent.
6. Practice with Different Types of Reactions
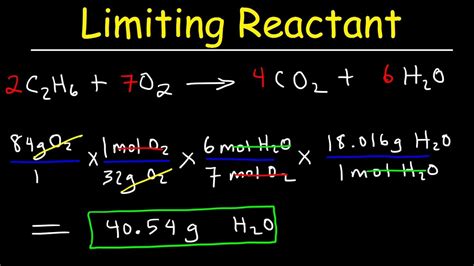
To become proficient in stoichiometry limiting reagent, you need to practice with different types of reactions, such as:
- Combustion reactions
- Synthesis reactions
- Decomposition reactions
- Replacement reactions
- Double displacement reactions
Practice solving problems using different types of reactions to become confident in your ability to determine the limiting reagent.
| Reaction Type | Example Reaction |
|---|---|
| Combustion Reaction | 2CH4 + 3O2 → 2CO2 + 3H2O |
| Synthesis Reaction | 2H2 + O2 → 2H2O |
| Decomposition Reaction | 2H2O → 2H2 + O2 |

Mastering stoichiometry limiting reagent takes time and practice. By following these six steps, you’ll become proficient in determining the limiting reagent and succeed in chemistry.
The ability to determine the limiting reagent is a critical skill in chemistry, and mastering it will help you succeed in your studies. Remember to practice regularly, use online resources, and seek help when needed. With persistence and dedication, you’ll become proficient in stoichiometry limiting reagent.
What is the limiting reagent in a reaction?
+
The limiting reagent is the reactant that is completely consumed in a reaction, thereby limiting the amount of product that can be formed.
How do I calculate the mole ratio of reactants and products?

+
To calculate the mole ratio, you need to divide the number of moles of each reactant and product by the smallest number of moles.
What is the ICE table method?
+
The ICE table method is a powerful tool for solving stoichiometry problems. It helps you organize your calculations and identify the limiting reagent.
Related Terms:
- Stoichiometry Limiting Reagent Worksheet
- Stoichiometry limiting reagent worksheet pdf
- Gas stoichiometry limiting reagent worksheet
- limiting reagent calculations worksheet
- limiting reagent worksheet pdf
- limiting reagent problems chemistry