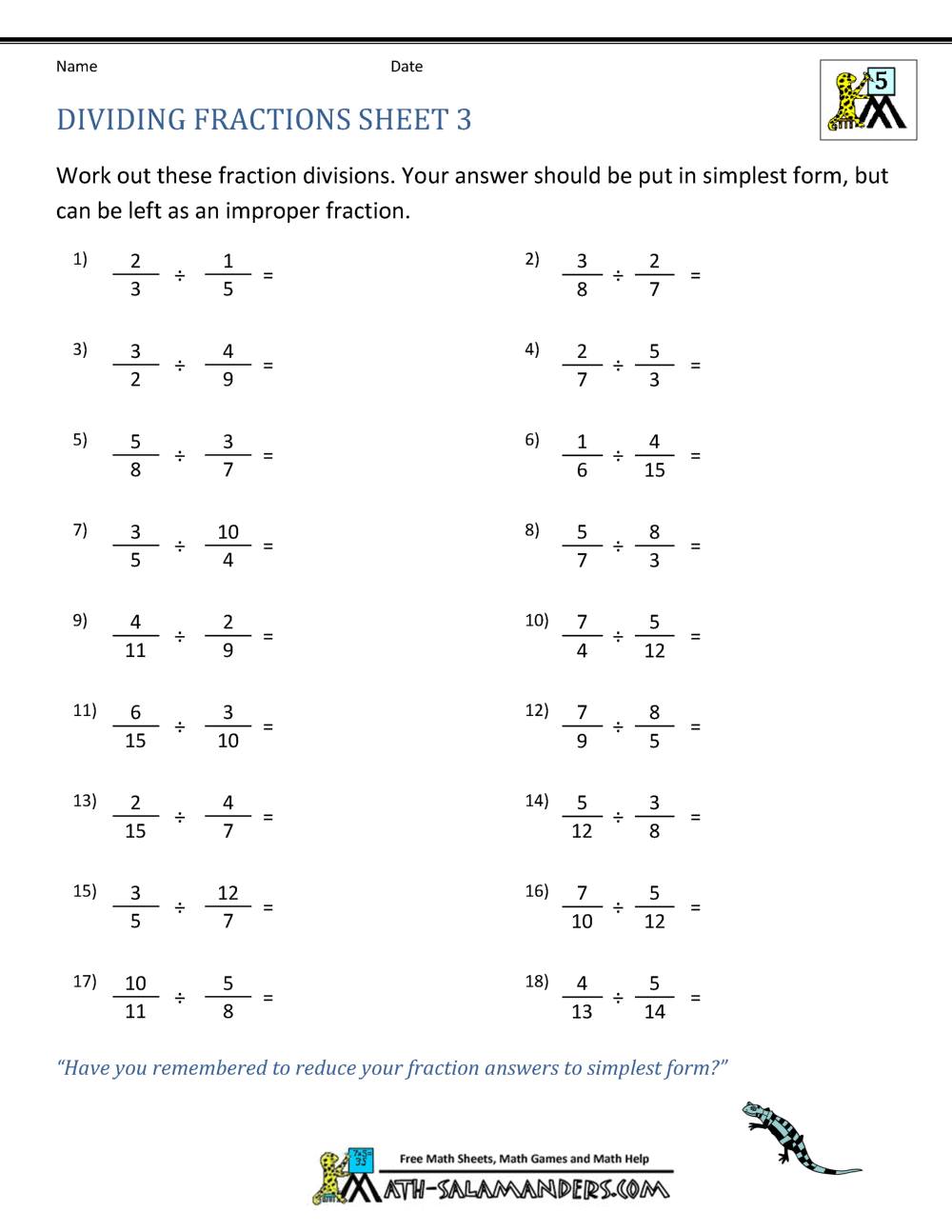Colloids, Suspensions & Solutions: Clear Guide & Worksheet

In the realm of physical chemistry, understanding the difference between colloids, suspensions, and solutions is fundamental. These systems play crucial roles in various fields, from industrial applications to everyday phenomena. Let's delve into each type, exploring their unique characteristics, properties, and how they behave differently under different conditions.
What are Colloids?

Colloids are heterogeneous mixtures where one substance is dispersed as very fine particles or droplets in another substance. The particles range in size from about 1 nm to 1000 nm, which is large enough to scatter light but not settle under gravity. Here are some key features:
- Stability: Colloidal systems are stable over time because the particles do not settle due to Brownian motion and electrostatic repulsion.
- Tyndall Effect: The scattering of light by colloidal particles, known as the Tyndall effect, is visible to the naked eye.
- Examples: Milk, fog, and gels like jelly.
Types of Colloids
| Dispersed Phase | Dispersion Medium | Example |
|---|---|---|
| Liquid | Gas | Fog, Aerosol sprays |
| Solid | Gas | Smoke, Dust in air |
| Gas | Liquid | Foam, Whipped cream |
| Liquid | Liquid | Emulsion, Milk |
| Solid | Liquid | Sol, Paint |
| Solid | Solid | Ruby glass, Pearl |

🔬 Note: Although colloids appear homogeneous to the naked eye, they are technically heterogeneous at a molecular level.
What are Suspensions?

Suspensions consist of larger particles than colloids, typically over 1000 nm, dispersed in a liquid medium where these particles settle under gravity due to their size and weight. Here are the defining characteristics:
- Settling: Over time, suspended particles will settle to the bottom due to gravitational forces.
- Opacity: Suspensions are often opaque because of light scattering by larger particles.
- Examples: Sand in water, chalk in water.
The properties of suspensions differ significantly from those of colloids:
- Can be filtered: You can use a filter paper to separate the solids from the liquid.
- Non-spontaneous dispersal: Particles require mechanical agitation to remain dispersed.
What are Solutions?
A solution is a homogeneous mixture of two or more substances, where one substance (the solute) is dissolved in another (the solvent). Here are some key points:
- Particle Size: In solutions, the particle size of solutes is at the ionic or molecular level, usually less than 1 nm.
- Transparency: Solutions are typically transparent or translucent due to the small size of solute particles.
- Examples: Salt water, sugar solution.
Solutions are stable and particles will not settle under gravity. They can also be classified based on:
- Concentration: Dilute, concentrated, or saturated.
- Electrolytes: Conduct electricity if dissolved in water.
🧪 Note: While all solutions are homogeneous at a macroscopic level, some can exhibit varying concentrations in microenvironments.
Worksheet Guide for Colloids, Suspensions, and Solutions

This section will provide a structured approach to help students understand and differentiate between these mixtures through practical examples and activities:
Experiment: Tyndall Effect

- Shine a laser pointer through a beaker of a colloid like milk, a suspension like chalk water, and a solution like sugar water. Observe and note the scattering of light.
Key Observations:
- Milk: Will exhibit the Tyndall effect.
- Chalk Water: Appears cloudy, and the effect is weak or absent due to particle size.
- Sugar Water: Will not show the Tyndall effect as it's transparent.
Comparing Properties

| Property | Colloid | Suspension | Solution |
|---|---|---|---|
| Particle Size | 1 nm to 1000 nm | Over 1000 nm | Less than 1 nm |
| Stability | Stable over time | Particles settle over time | Stable, no settling |
| Filtration | Passes through filter paper | Can be filtered | Not filterable |
| Appearance | Scatters light, appears turbid | Cloudy or opaque | Transparent or translucent |
To conclude, understanding colloids, suspensions, and solutions is not just academic but has practical implications in fields like food science, pharmaceuticals, and even environmental science. These systems demonstrate how the size and interaction of particles can profoundly influence the physical behavior of mixtures. Recognizing the differences and using these concepts can lead to innovations in product development, analytical techniques, and the study of natural phenomena. From industrial applications to everyday life, the knowledge of these mixtures helps us understand why certain mixtures behave the way they do, how to stabilize products, and how environmental factors might affect them. Whether it's the stability of your favorite gel, the sedimentation of your paint, or the transparency of your drinking water, the principles we've discussed govern their properties and behaviors.
What is the main difference between a colloid and a suspension?

+
The primary difference lies in particle size. Colloids have particles from 1 nm to 1000 nm, which remain suspended due to Brownian motion, whereas suspensions have particles larger than 1000 nm, which settle over time due to gravity.
Can colloids be filtered like suspensions?

+
No, colloids cannot be filtered by typical filter paper as the particles are too small to be caught, whereas suspensions can be filtered because of their larger particle size.
Why does milk scatter light while water doesn’t?

+
Milk contains colloidal casein micelles which scatter light, causing the Tyndall effect. Water, being a solution, does not have particles large enough to scatter light effectively, so it remains transparent.