Master Ionic Compounds: Naming Practice Worksheet

Ionic compounds, formed from the attraction between positively and negatively charged ions, play a crucial role in various chemical processes and everyday applications. In the world of chemistry, the ability to name ionic compounds accurately is essential for both understanding and effective communication within the scientific community. This blog post delves into the naming of ionic compounds, providing a comprehensive practice worksheet, guidelines, and tips to master this vital aspect of chemistry. Let's begin with a clear understanding of how to name these compounds.
Understanding Ionic Compounds

Ionic compounds are composed of ions—charged particles formed when atoms gain or lose electrons. These ions are held together by ionic bonds, which are electrostatic attractions due to the opposite charges on the ions.
Key Points to Understand:
- Cations are positively charged ions, resulting from atoms losing electrons.
- Anions are negatively charged ions, formed when atoms gain electrons.
- The formula of an ionic compound reflects the simplest ratio of the ions, known as the empirical formula.
Rules for Naming Ionic Compounds

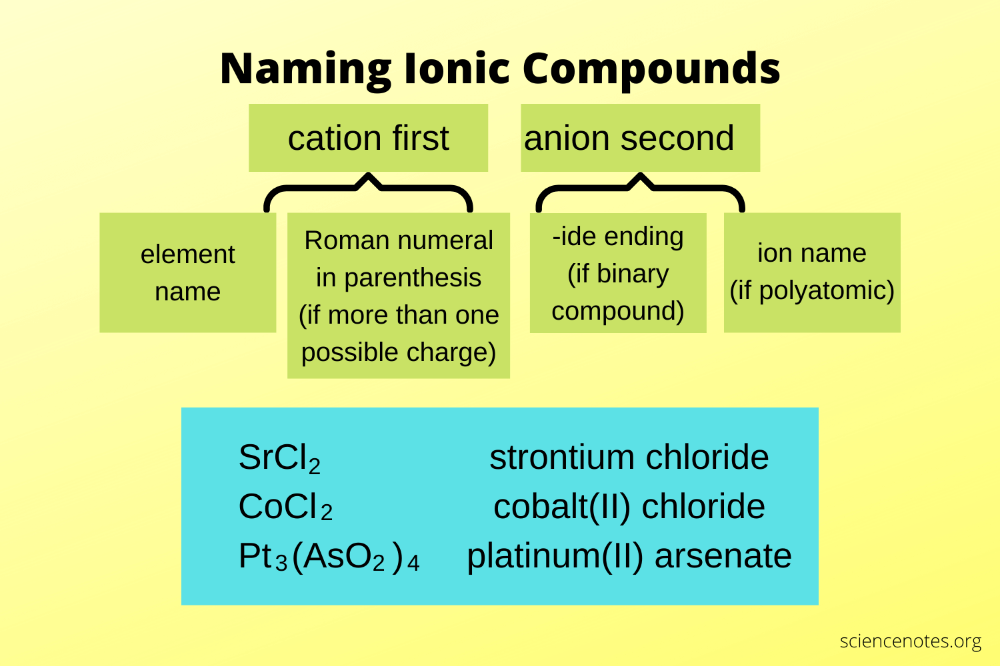
Naming ionic compounds involves following several straightforward rules:
- The cation name comes first. For metals that can have multiple charges (like iron, copper, etc.), the charge is specified using Roman numerals.
- The anion name comes second. For monatomic anions, the element name ends in "-ide". Polyatomic anions have specific names.
- When a metal can form multiple charges, use Roman numerals in parentheses after the metal's name to indicate the charge.
Examples:

- NaCl: Sodium chloride
- CuO (copper is +2): Copper(II) oxide
- Fe₂O₃ (iron is +3): Iron(III) oxide
- MgSO₄: Magnesium sulfate
Practice Worksheet: Naming Ionic Compounds

Here is a naming practice worksheet to help solidify your understanding of naming ionic compounds:
| Formula | Name | Charge on Metal (if applicable) |
|---|---|---|
| KCl | ||
| MgI₂ | ||
| Cu₂CO₃ | ||
| Al₂(SO₄)₃ | ||
| FeCl₃ |

Answers to Worksheet:

- KCl: Potassium chloride
- MgI₂: Magnesium iodide
- Cu₂CO₃: Copper(I) carbonate
- Al₂(SO₄)₃: Aluminum sulfate
- FeCl₃: Iron(III) chloride
⚠️ Note: When dealing with compounds containing transition metals, always confirm the metal's charge using the periodic table or known compounds.
Tips for Mastering Ionic Compound Nomenclature

To truly excel in naming ionic compounds, consider the following tips:
- Memorize polyatomic ions: Understand the names, formulas, and charges of common polyatomic ions.
- Recognize patterns: Recognize patterns in the periodic table to predict ion formation.
- Practice with transition metals: Transition metals often have multiple charges; get comfortable identifying which charge they are exhibiting in different compounds.
- Use flashcards or online resources: Visual aids and interactive tools can help reinforce memory.
📝 Note: When faced with ambiguous metals, remember to use the charge from the non-metal anion to deduce the cation's charge.
Wrapping Up

Having explored the nuances of naming ionic compounds, it's clear that this skill is not just about memorization but also about understanding the chemical behavior of elements. From the straightforward sodium chloride to the more complex iron oxide, ionic compounds span the chemical world, playing vital roles in industries, medicine, and everyday life. By mastering the principles outlined here, you are well on your way to confidently deciphering and naming the vast array of ionic compounds you might encounter in your studies or professional life.
What are the main differences between ionic and covalent compounds?

+
Ionic compounds are formed through ionic bonds where electrons are transferred, resulting in charged ions. Covalent compounds, on the other hand, involve sharing of electrons to form stable molecules without ion formation.
Why is it necessary to specify the charge for some transition metals?

+
Transition metals like iron, copper, or chromium can form multiple charges. Specifying the charge with Roman numerals helps to differentiate between different compounds formed by the same elements.
How can I quickly recognize polyatomic ions?

+
Regularly practicing and associating common polyatomic ions with their charges, names, and formulas will help. Flashcards and quizzes can be effective tools for memorization.
What are some practical applications of understanding ionic compounds?
+
Knowledge of ionic compounds aids in material science, pharmaceuticals, food chemistry, environmental science, and even basic kitchen activities like understanding salt’s role in cooking.