Rate Laws Worksheet Answers: Master Chemical Kinetics Easily

Understanding the principles behind chemical kinetics is crucial for anyone involved in the realm of chemistry, whether you are a student, educator, or professional. Chemical kinetics explores the rates of chemical reactions, how these rates are influenced, and the pathways by which reactions proceed. This guide will delve into rate laws, helping you to master the intricacies of how reactions occur at varying speeds.
What are Rate Laws?
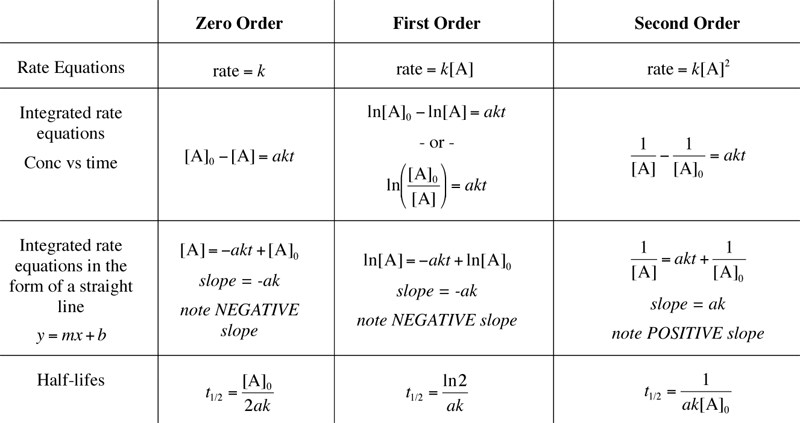
Rate laws are mathematical expressions used to predict the rate of a chemical reaction. They provide insight into the relationship between the reaction rate and the concentration of reactants. The general form of a rate law for a reaction like aA + bB → cC + dD is:
Rate = k[A]m[B]n
- k: The rate constant, which reflects the reaction’s intrinsic speed at specific conditions like temperature.
- [A], [B]: The molar concentrations of reactants A and B.
- m, n: The reaction orders with respect to A and B, respectively, which indicate how the rate changes with changes in concentration.
Determining Rate Laws Experimentally

Rate laws aren’t derived theoretically; they are determined through experimental observation. Here’s a basic method to find them:
- Initial Rate Method: Measure the rate of the reaction at various starting concentrations of reactants to observe how initial rates vary.
- Method of Isolation: Isolate the effect of changing one reactant at a time by keeping others constant.
- Graphical Methods: Plotting concentration vs. time can sometimes reveal the reaction order.
💡 Note: The experimental approach often involves fitting the data to find the best match for the rate law equation.
Example Problem: Find the Rate Law

Consider a reaction where reactant A is varied and the initial rate is observed:
| Experiment | A | Rate (M/s) |
|---|---|---|
| 1 | 0.10 | 0.020 |
| 2 | 0.20 | 0.080 |
| 3 | 0.30 | 0.180 |

To find the rate law, you can apply the following steps:
- Compare rates for experiments where only [A] changes.
- Use the ratio method to determine the reaction order with respect to A:
Let's use the rates from experiments 1 and 2:
Rate₂ / Rate₁ = (k[0.2]m) / (k[0.1]m) = 0.080 / 0.020 = 4
This implies:
(2)m = 4; thus, m = 2 (The reaction is second-order with respect to A)
📝 Note: For real data, use logarithms to solve for exponents or utilize graphing software for better accuracy.
How to Apply Rate Laws in Real-Life Scenarios
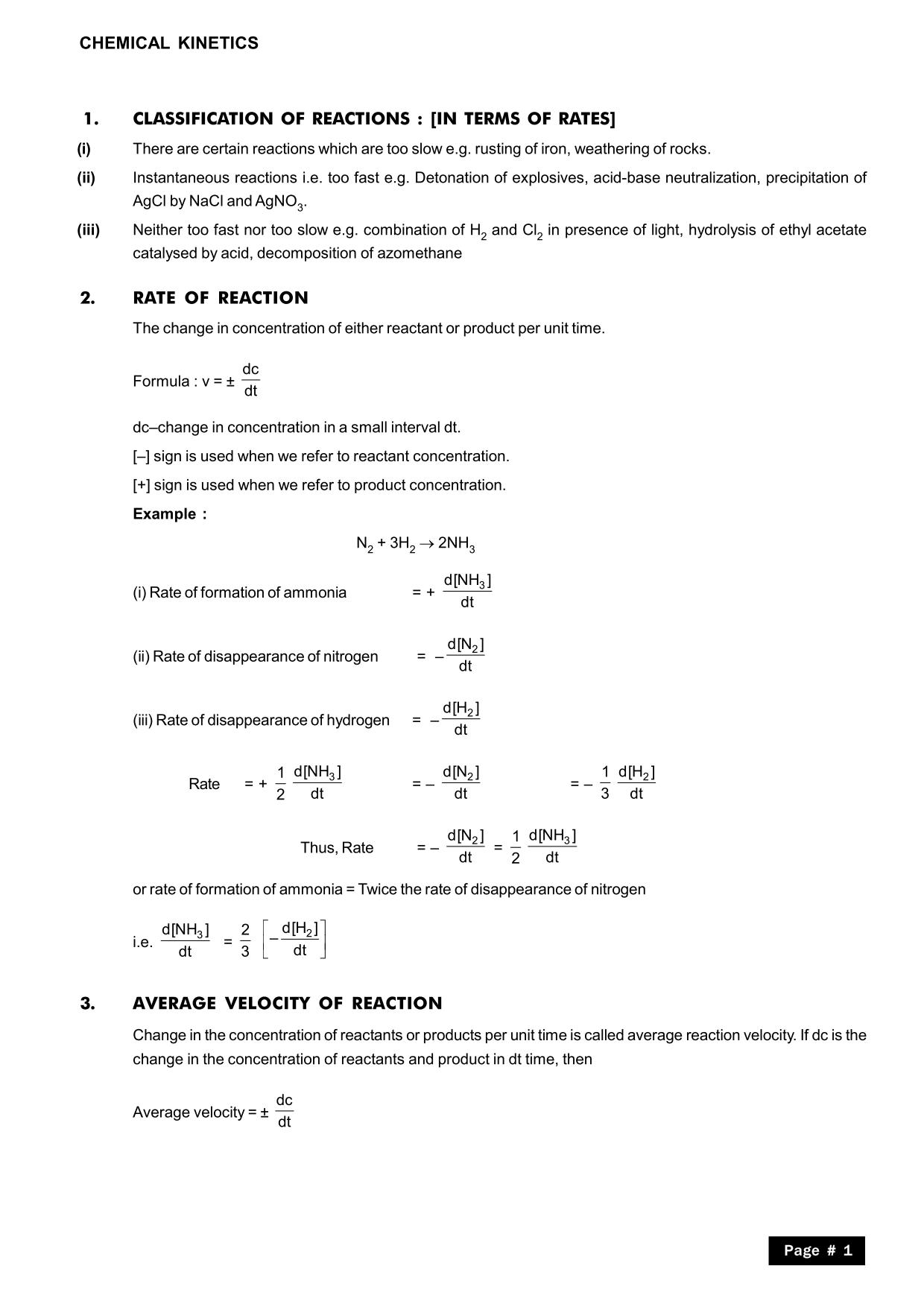
- Reaction Mechanisms: Understanding rate laws helps in deducing the sequence of elementary steps in a reaction mechanism.
- Pharmaceutical Industry: Drug design and stability assessments require an understanding of how reactants break down or form.
- Environmental Chemistry: Pollution rates, degradation of pollutants, and the impact of temperature changes can be predicted using rate laws.
Common Pitfalls in Rate Law Calculations

- Assuming the rate law from stoichiometry: Reaction order does not always correspond to the stoichiometric coefficients.
- Ignoring the Rate Constant’s Dependence on Temperature: The rate constant k changes with temperature according to the Arrhenius equation.
- Overlooking Complex Reactions: Not all reactions follow simple rate laws, especially when intermediates are involved.
📈 Note: Always validate your rate laws with experimental data to avoid these pitfalls.
Summary

To wrap up, mastering rate laws in chemical kinetics is about understanding how the rate of a reaction depends on reactant concentrations, the reaction order, and how these can be determined experimentally. Here are the key takeaways:
- Rate laws provide a mathematical model to predict reaction rates.
- Experimental data is crucial for determining rate laws accurately.
- The rate constant and reaction orders are not constant but depend on specific conditions like temperature.
- Understanding rate laws has practical applications in fields like drug discovery, environmental science, and industrial chemistry.
How do I know if my rate law is correct?

+
Your rate law is correct if it accurately predicts the reaction rates for different sets of initial concentrations of reactants and matches experimental data. If the rate law does not match, consider re-evaluating your data collection or the reaction conditions.
Why is the rate law important for predicting reaction rates?

+
The rate law gives insights into how fast or slow a reaction will proceed based on the concentration of reactants. This is crucial for optimizing reaction conditions and understanding reaction mechanisms.
Can rate laws change with temperature?

+
While the form of the rate law (reaction order) generally does not change with temperature, the rate constant k does increase exponentially with rising temperature, following the Arrhenius equation.



