5 Easy Steps to Master Equilibrium Expressions

Mastering equilibrium expressions is pivotal for students, scientists, and professionals in chemistry, as it allows a deeper understanding of chemical reactions and how they reach a state of balance. The concept of chemical equilibrium applies to reactions where the rates of the forward and reverse reactions become equal, leading to a state where the concentrations of reactants and products remain constant over time. This guide will take you through five easy steps to understand, write, and solve equilibrium expressions effectively.
Step 1: Understanding Equilibrium

Chemical equilibrium occurs when the concentrations of reactants and products do not change with time in a closed system. This doesn’t mean that the reactions have stopped, but rather that they are proceeding at equal rates in both directions.
- Le Chatelier’s Principle: This principle describes how a system at equilibrium responds to changes in temperature, pressure, or concentration.
- Dynamic Equilibrium: The reactions continue to occur, but at an identical rate, maintaining a constant ratio of reactants to products.
Step 2: Writing Equilibrium Expressions

Equilibrium expressions are mathematical equations that describe the relationship between the concentrations of reactants and products at equilibrium:
The Law of Mass Action
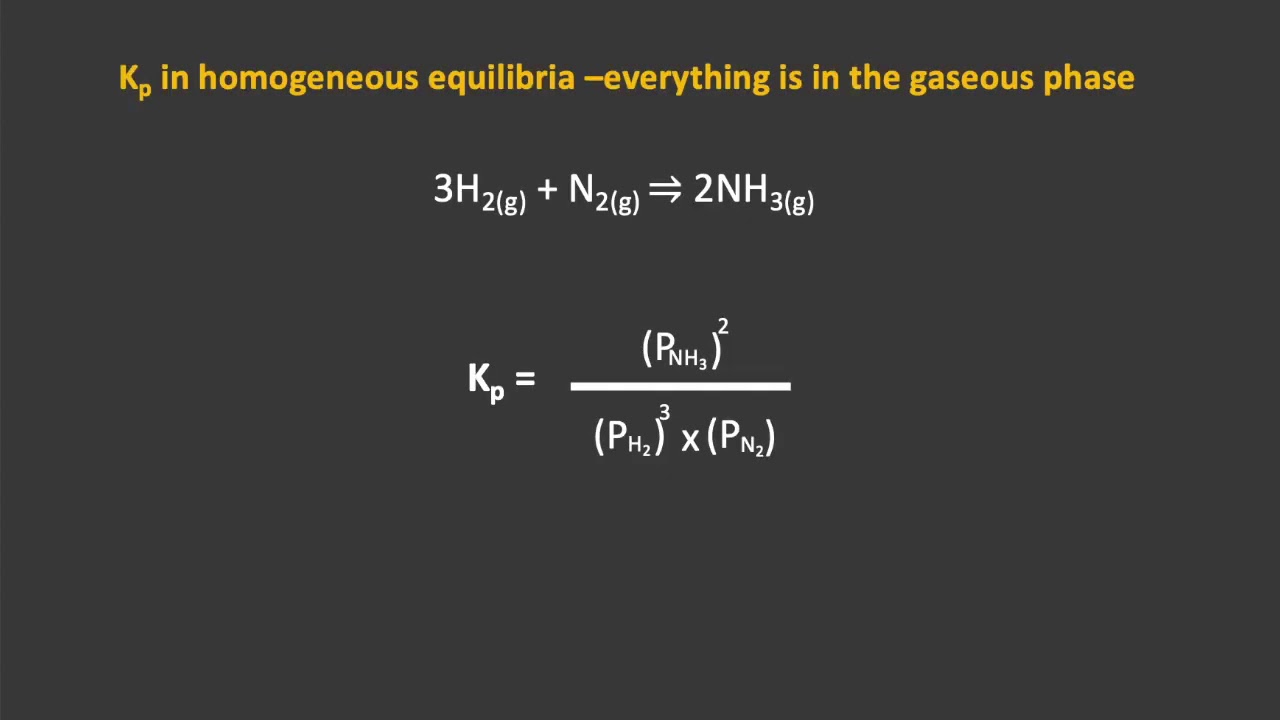
To write an equilibrium expression, follow these steps:
- Identify Reactants and Products: Note all species in your reaction equation, including their coefficients.
- Set Up the Expression: Write the concentration of products raised to their stoichiometric coefficients on the top, and the reactants at the bottom, also raised to their coefficients.
- Exclude Pure Solids and Liquids: Since their concentrations do not change, they are not included in the equilibrium expression.
For example, for the general reaction:
A + B ⇌ C + D
The equilibrium expression (Kc) would be:
Kc = ([C][D])/([A][B])
⚠️ Note: The expression does not include the coefficients in the equation when calculating concentration terms, only the products' and reactants' concentrations themselves.
Step 3: Solving Equilibrium Problems

Solving for equilibrium concentrations involves a few key steps:
- Set Up an ICE Table: ICE stands for Initial, Change, and Equilibrium. This table helps track concentration changes throughout the reaction.
- Substitution and Solving: Substitute the equilibrium values into the equilibrium expression and solve for the unknown concentrations.
Here’s an example of an ICE table for the reaction A + B ⇌ C + D:
| Species | Initial Concentration | Change | Equilibrium Concentration |
|---|---|---|---|
| A | 0.1 M | -x | 0.1-x M |
| B | 0.2 M | -x | 0.2-x M |
| C | 0.0 M | +x | x M |
| D | 0.0 M | +x | x M |

✨ Note: Always double-check the units of concentration used in your ICE table and ensure consistency throughout your calculations.
Step 4: Applying Equilibrium Expressions

Once you can write and solve equilibrium expressions, you can apply this knowledge to various real-world scenarios:
- Acid-Base Chemistry: Calculate the pH of weak acids or bases using the acid or base dissociation constants.
- Solubility Product: Determine solubility of sparingly soluble salts.
- Reaction Quotient (Q): Predict the direction in which a reaction will shift to reach equilibrium.
💡 Note: The reaction quotient, Q, is essentially the equilibrium expression using initial concentrations or pressures, and it indicates if the system is at, above, or below equilibrium.
Step 5: Interpreting Equilibrium Constants
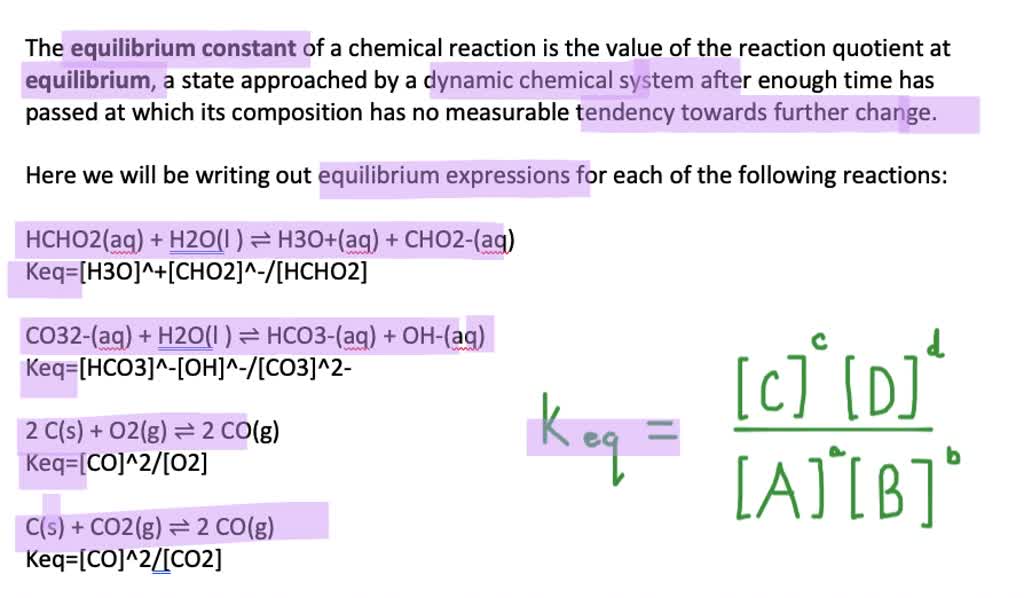
The equilibrium constant, K, can reveal much about a reaction:
- Large K Values: Indicate that products are favored at equilibrium.
- Small K Values: Suggest that reactants are favored at equilibrium.
- Units: Equilibrium constants have units derived from the ratio of product to reactant concentrations. These units are often ignored for simplicity.
📝 Note: Equilibrium constants are only dependent on temperature, not on concentration, pressure, or volume of the system.
By mastering these five steps, you'll gain a strong foundation in chemical equilibrium, enabling you to understand, predict, and control reactions in various contexts. The ability to manipulate and interpret equilibrium expressions is crucial for advancing in chemistry, from basic understanding to complex problem-solving and experimental design.
What is the difference between Kc and Kp?

+
Kc is the equilibrium constant based on molar concentrations, while Kp is based on partial pressures of gaseous components. The relationship between the two is Kp = Kc(RT)^Δn, where Δn is the change in the number of moles of gas in the reaction.
Why are solids and liquids excluded from equilibrium expressions?

+
Pure solids and liquids have a constant concentration, which doesn’t change during the reaction, so their concentrations are effectively incorporated into the equilibrium constant.
Can equilibrium expressions be used for every chemical reaction?

+
Equilibrium expressions are primarily used for reactions that can reach equilibrium, meaning those that are reversible. Reactions that go to completion or are extremely slow might not fit this model.
How does changing the concentration of reactants affect the equilibrium?

+
According to Le Chatelier’s principle, increasing the concentration of reactants will shift the equilibrium towards the products, while decreasing it will shift it towards the reactants. However, this only changes the equilibrium concentration, not the equilibrium constant itself.



