Protons Neutrons Electrons Worksheet Answers Explained

Understanding Protons, Neutrons, and Electrons
Atoms are the fundamental building blocks of matter, and they consist of even smaller subatomic particles known as protons, neutrons, and electrons. Each plays a crucial role in determining the properties of an element, and understanding their characteristics can be key to mastering chemistry. In this worksheet, we will explore how these particles interact and contribute to the atom’s structure.
What Are Protons?

Protons are positively charged particles found within the nucleus of an atom. Here are some important points about protons:
- Charge: Protons have a positive electric charge (+1).
- Location: They are located in the nucleus of the atom.
- Mass: A proton has a mass of approximately 1.67262 x 10-27 kilograms, which is roughly 1 atomic mass unit (amu).
- Influence: The number of protons in an atom defines the atomic number and thus, the element’s identity.
What Are Neutrons?

Neutrons are electrically neutral particles that reside in the nucleus alongside protons. Here’s a brief rundown:
- Charge: Neutrons have no electrical charge (neutral).
- Location: They are also found in the nucleus.
- Mass: Neutrons are slightly heavier than protons, with a mass of about 1.67493 x 10-27 kg, or about 1 amu.
- Influence: The number of neutrons can affect the mass of an atom and its stability. Different numbers of neutrons in the same element result in isotopes.
What Are Electrons?

Electrons are negatively charged particles that orbit the nucleus in various energy levels or shells. Here are the key aspects of electrons:
- Charge: Electrons have a negative electric charge (-1).
- Location: They orbit the nucleus in electron shells or orbits.
- Mass: Electrons have a much smaller mass than protons and neutrons, with a mass of approximately 9.10938 x 10-31 kg.
- Influence: Electrons are involved in chemical reactions, bonding, and the electrical properties of substances.
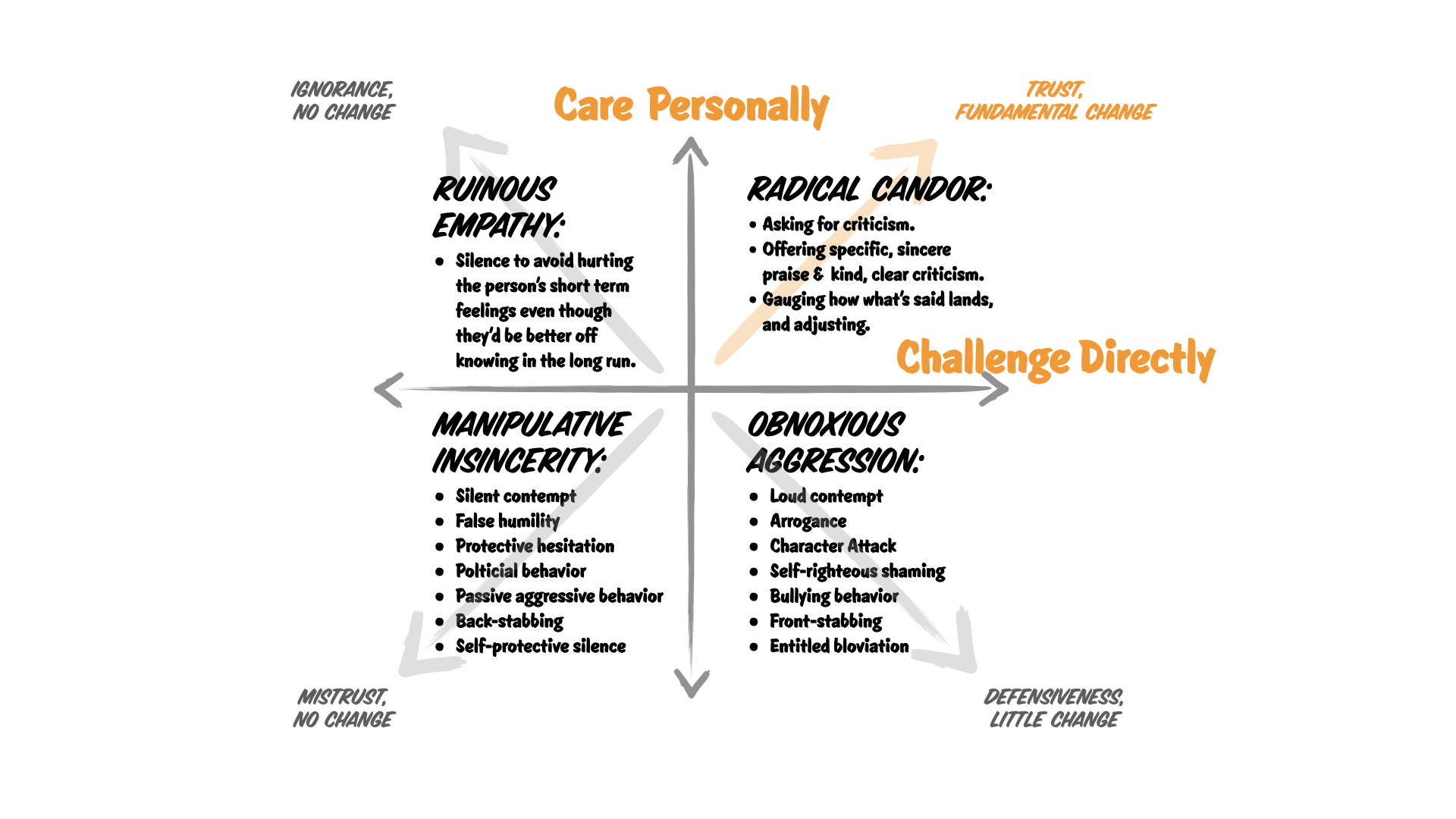
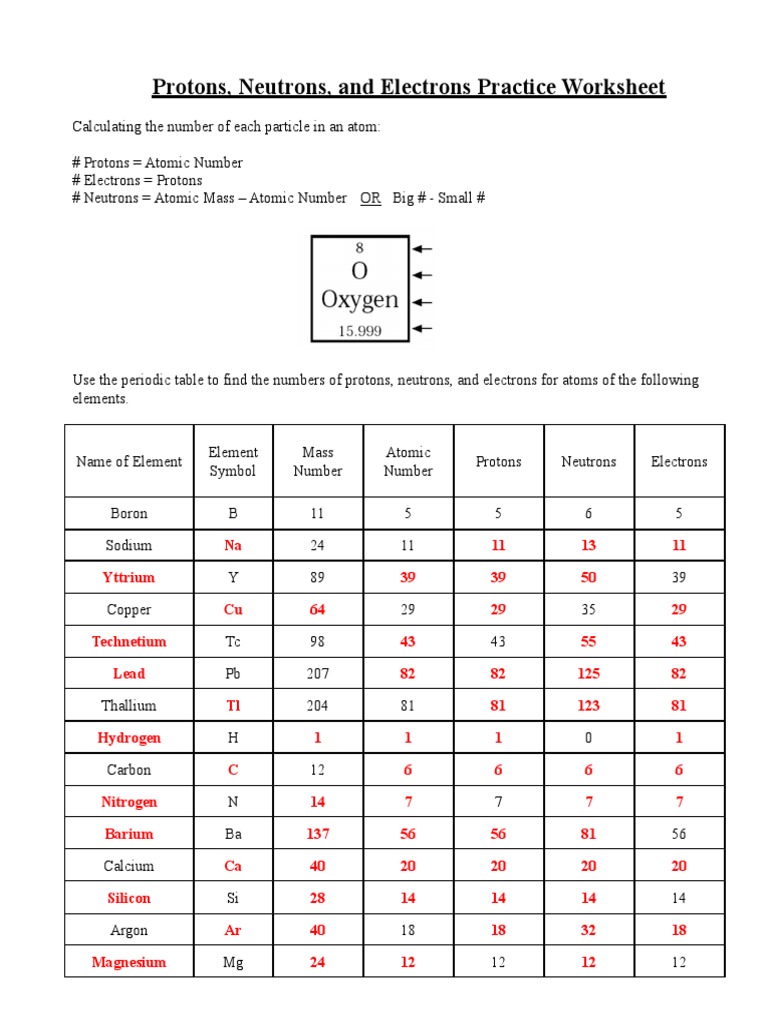
Worksheet Answers

Now let’s delve into some common questions and answers found in a typical protons, neutrons, and electrons worksheet:
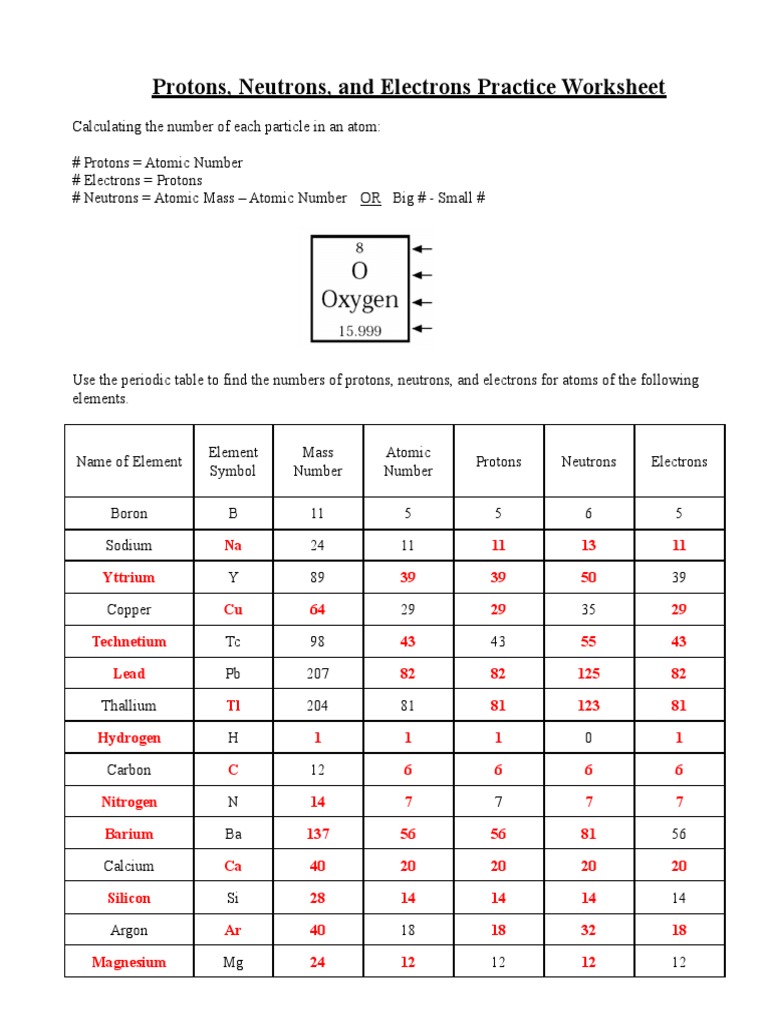
1. Atomic Number and Mass Number

Consider an atom of Neon-20:
- The atomic number of Neon is 10, which tells us Neon has 10 protons.
- The mass number (20) is the total of protons and neutrons in the nucleus.
- Therefore, to find the number of neutrons:
Neutron Number = Mass Number - Atomic Number= 20 - 10 = 10 neutrons.
2. Electron Configuration

Take the example of Carbon-12:
- Carbon has an atomic number of 6, so it has 6 protons.
- In its neutral state, Carbon has 6 electrons, which are distributed as follows:
- First shell (K-shell): 2 electrons
- Second shell (L-shell): 4 electrons
3. Isotopes
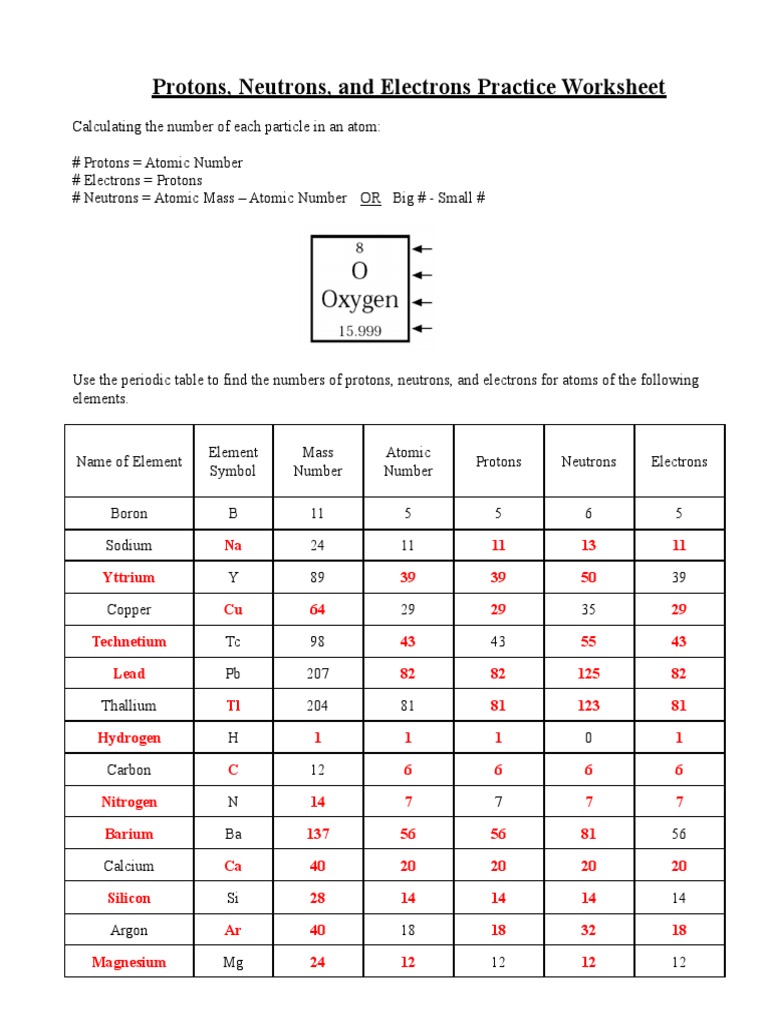
Consider the isotopes of Hydrogen:
- Hydrogen-1: 1 proton, 0 neutrons, 1 electron
- Deuterium (Hydrogen-2): 1 proton, 1 neutron, 1 electron
- Tritium (Hydrogen-3): 1 proton, 2 neutrons, 1 electron
These isotopes all have the same number of protons but differ in the number of neutrons, which affects their mass and stability.
⚠️ Note: All atoms of a given element have the same number of protons, but the number of neutrons can vary, leading to different isotopes.
4. Ion Formation
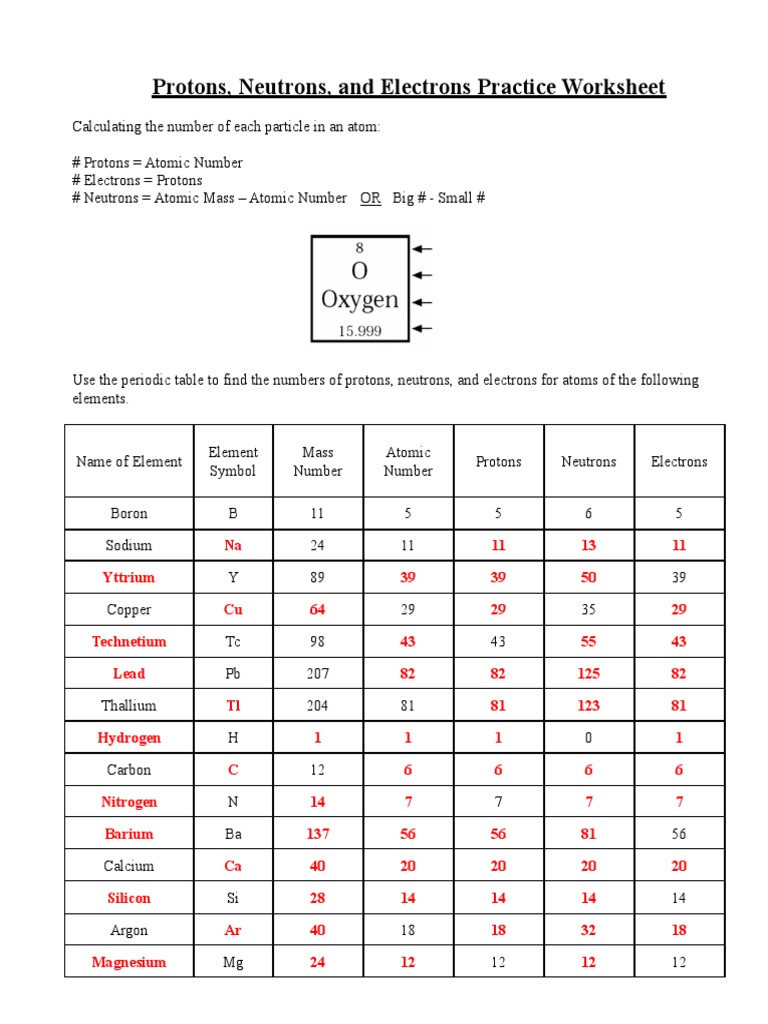
When atoms gain or lose electrons, they form ions:
- If an atom loses an electron, it becomes positively charged (cation). For example, Na+ (Sodium cation) has 11 protons and 10 electrons.
- If an atom gains an electron, it becomes negatively charged (anion). For example, Cl- (Chloride anion) has 17 protons and 18 electrons.
5. Mass Calculation
The relative atomic mass or atomic weight of an element accounts for all its isotopes:
| Isotope | Mass Number | Natural Abundance | Contribution to Atomic Weight |
|---|---|---|---|
| Carbon-12 | 12 | 98.93% | 12 * 0.9893 = 11.8716 |
| Carbon-13 | 13 | 1.07% | 13 * 0.0107 = 0.1391 |
| Carbon-14 | 14 | negligible | 14 * 0 = 0 |

The approximate atomic weight of Carbon would be the sum of the contributions: 11.8716 + 0.1391 = 12.0107 amu.
🔍 Note: The atomic weight listed on a periodic table is an average, taking into account the natural abundance of each isotope.
In wrapping up this exploration of protons, neutrons, and electrons, we have covered the basics of atomic structure and how these particles contribute to an atom’s identity, behavior, and interactions. Understanding these fundamental components allows us to grasp the principles behind atomic theory, chemical bonding, and nuclear reactions. By identifying the number of protons, neutrons, and electrons in an atom, we unlock the secrets of the periodic table, isotope variations, and the dynamics of ionic formation. This knowledge forms the bedrock upon which many scientific disciplines, from chemistry to physics to biology, are built.
What determines an element’s atomic number?
+
The atomic number is determined by the number of protons in the nucleus of an atom. Each element has a unique atomic number, which defines its identity.
How do isotopes differ from one another?
+
Isotopes are variants of an element with the same number of protons but different numbers of neutrons. This difference in neutron count leads to variations in mass number and often in nuclear stability.
What happens when an atom loses or gains electrons?
+When an atom loses electrons, it becomes a positively charged ion (cation). Conversely, when it gains electrons, it becomes a negatively charged ion (anion). These ions play crucial roles in chemical reactions and bonding.