5 Tips to Master Worksheet Reaction Rates

When it comes to mastering worksheet reaction rates in chemistry, understanding how to analyze and predict how fast reactions occur is not just a study tool, it's an essential skill. Rates of reactions are crucial in a variety of applications, from the optimization of industrial processes to the development of pharmaceuticals. Here are five tips designed to help you excel in understanding and solving reaction rates problems effectively.
1. Grasp the Basics of Rate Laws


First and foremost, get comfortable with the basics of rate laws:
- Rate law: Understand that the rate of a reaction is dependent on the concentration of reactants. The general form is rate = k[A]m[B]n, where k is the rate constant, and m and n are the orders with respect to A and B, respectively.
- Reaction order: The sum of exponents (m+n) in the rate law is the overall order of the reaction.
- Zero-, first-, and second-order kinetics: These describe how the rate changes with changes in the concentration of reactants.
⚠️ Note: Always make sure to differentiate between the rate constant (k), which is temperature dependent, and the order of reaction which is determined by the rate law.
2. Utilize the Integrated Rate Law for Rate Calculations

| Order of Reaction | Integrated Rate Law | Linear Plot |
|---|---|---|
| 0 | [A] = -kt + [A]0 | [A] vs. time |
| 1 | ln[A] = -kt + ln[A]0 | ln[A] vs. time |
| 2 | 1/[A] = kt + 1/[A]0 | 1/[A] vs. time |

By understanding the integrated forms, you can plot data to determine the reaction order:
- For zero-order reactions, plot the concentration of the reactant against time.
- First-order kinetics are linear when you plot the natural log of concentration vs. time.
- Second-order kinetics yield a straight line when plotting the inverse of concentration vs. time.
🎯 Note: These plots can be used to confirm the order of a reaction visually, making it easier to analyze complex data sets.
3. Master the Method of Initial Rates
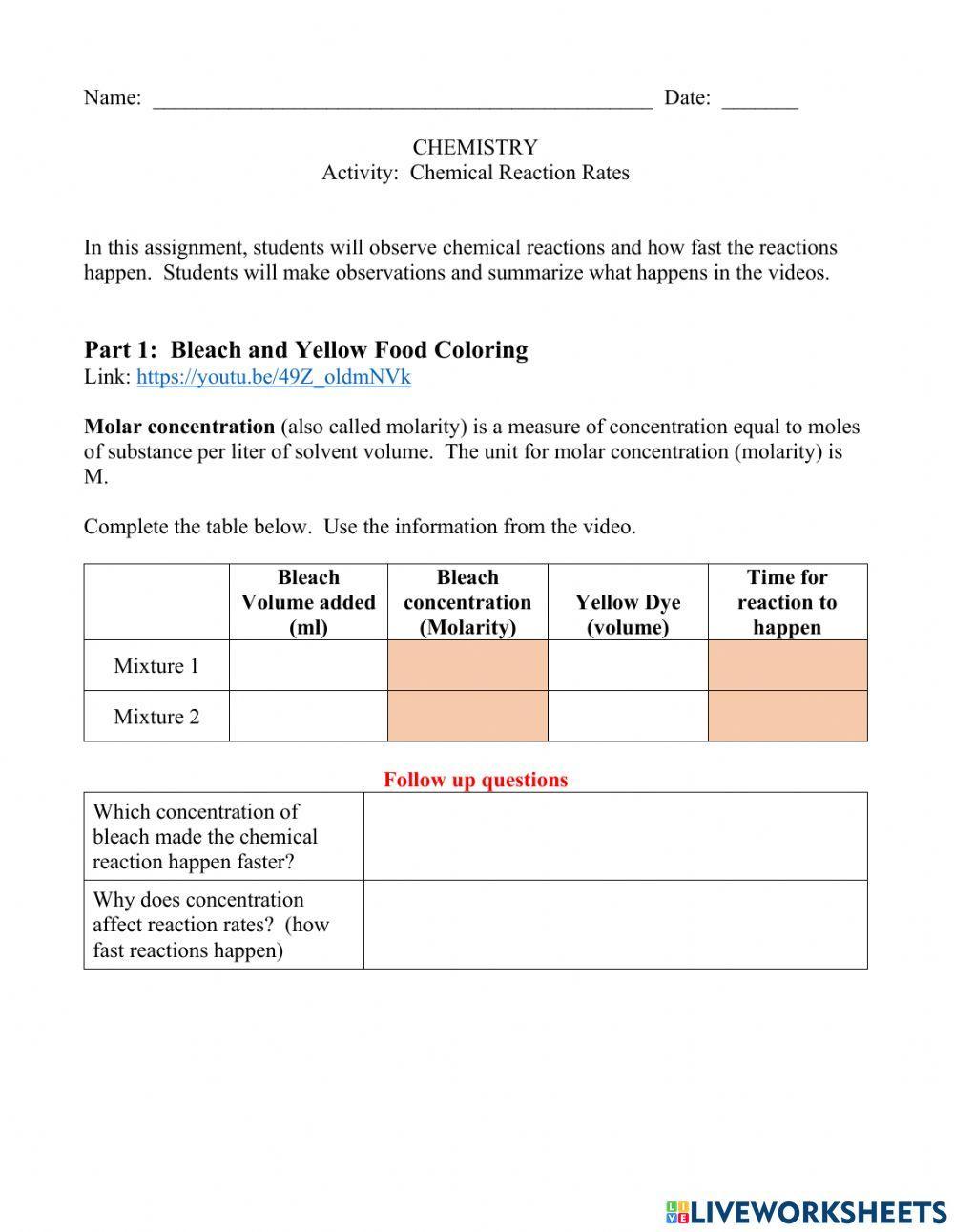
This method helps determine the rate law and order of reaction by measuring initial rates at different concentrations:
- Change the concentration of one reactant while holding others constant to see how the rate changes.
- Calculate the ratio of the rates for two experiments where the concentration of the reactant has changed by the same ratio.
📝 Note: When comparing experiments, ensure that only one reactant's concentration is altered to avoid complications in analysis.
4. Understand Catalysts and Activation Energy


A reaction’s rate can also be influenced by:
- Catalysts: They lower the activation energy without being consumed in the reaction, thereby speeding it up.
- Activation energy (Ea): The energy barrier reactants must overcome to become products. Lowering this barrier increases the rate.
5. Practice with Real-World Applications

Connect theory to real-life scenarios:
- Industrial processes often depend on optimizing reaction rates for cost-effectiveness and environmental impact.
- Drug design in pharmaceuticals involves understanding and manipulating reaction rates to produce desired compounds efficiently.
- Understand how rate considerations play a part in environmental chemistry, food preservation, and energy production.
🧪 Note: Applying your knowledge to practical problems not only reinforces the theory but also highlights its importance in everyday applications.
In summary, mastering worksheet reaction rates involves a deep understanding of rate laws, integrated forms, initial rates, the effects of catalysts, and practical applications. By internalizing these concepts, you'll be able to solve problems with greater efficiency and understand the underlying kinetics of chemical reactions. Engaging with this knowledge through practice and application will not only help you in your studies but also prepare you for real-world scenarios where reaction rates play a crucial role.
Why is it important to understand reaction rates?

+
Understanding reaction rates helps in optimizing industrial processes, designing better pharmaceuticals, reducing environmental pollution, and managing chemical reactions for safety and efficiency.
How can I identify the order of a reaction?

+
You can identify the order by using the method of initial rates or plotting data against time to see if it matches the integrated rate laws for zero, first, or second order.
What is the role of a catalyst in reactions?

+
Catalysts accelerate reactions by lowering the activation energy, allowing the reaction to proceed faster without being consumed in the process.
What are some practical applications of studying reaction rates?

+
Practical applications include refining industrial processes for efficiency, drug discovery, environmental protection, and improving energy technologies like batteries and fuel cells.