5 Essential Tips for Potential Energy Diagram Worksheets
Creating Engaging Potential Energy Diagram Worksheets

When it comes to teaching the intricacies of potential energy and chemical reactions in physics and chemistry, potential energy diagrams serve as an invaluable visual tool. Whether you’re an educator crafting resources for your students or a student looking to master this concept, here are five essential tips for making the most out of potential energy diagram worksheets.
1. Understand the Basics Before Diving In

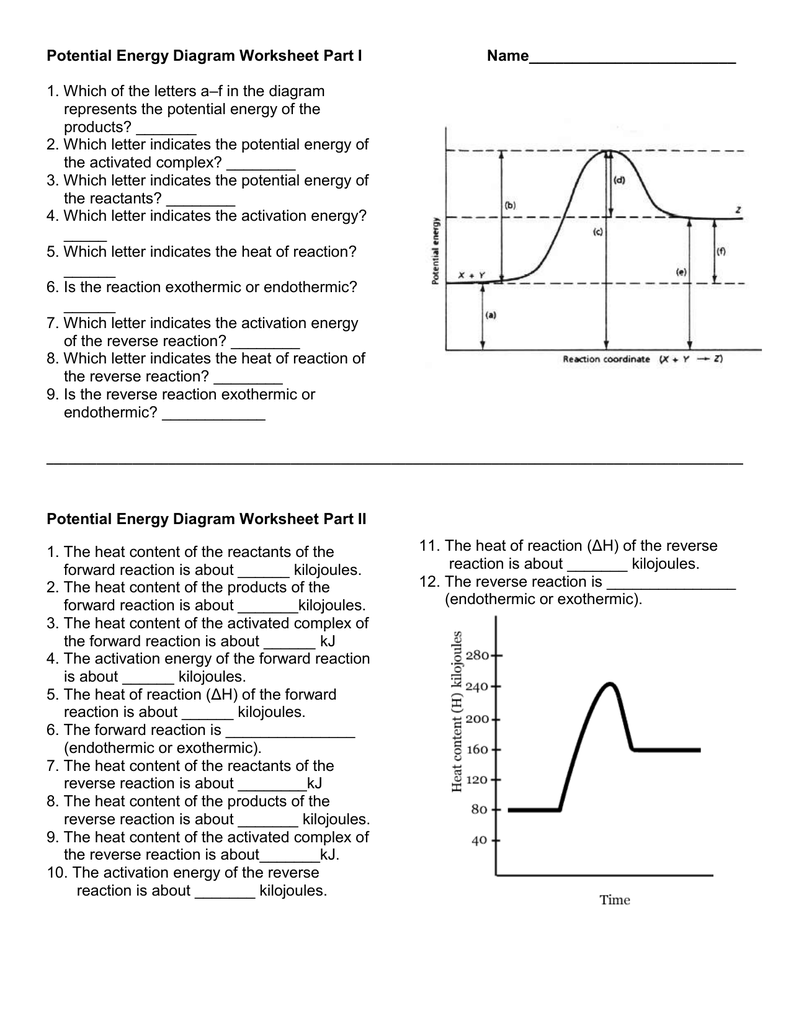
Before you start plotting points and drawing curves, ensure you have a solid grasp of what a potential energy diagram represents. A potential energy diagram illustrates how the potential energy of a system changes during a chemical reaction or physical process. Here are the core components:
- Reaction Coordinate (X-axis): This axis represents the progress of the reaction, from reactants to products.
- Potential Energy (Y-axis): Shows the amount of potential energy stored in the system at various stages of the reaction.
- Reactants and Products: These are represented by points on the diagram where energy levels start and end.
- Transition State: The peak of the curve represents the highest energy state the system passes through, known as the activated complex.
- Activation Energy: The energy needed to initiate the reaction is depicted as the height of the barrier from the reactant to the transition state.
By understanding these fundamentals, you can design worksheets that not only depict but also explain these concepts effectively.
2. Use Color Coding and Clarity in Presentation

Visual clarity can significantly enhance the understanding of complex diagrams. Here’s how to utilize color:
- Reactants and Products: Color them in different shades to distinguish them easily.
- Transition State and Activation Energy: Highlight these areas with contrasting colors to make them stand out.
- Curves: Use different line styles or colors for exothermic and endothermic reactions.
🎨 Note: While color-coding aids in understanding, ensure that the diagrams are still legible in black and white, for students with color vision deficiencies or when printing in monochrome.
3. Incorporate Real-life Examples

Potential energy diagrams aren’t abstract theories; they represent real-life scenarios. Here are some examples to include:
- Chemical reactions like the combustion of methane or rusting of iron.
- Physical changes such as stretching a rubber band or the expansion of a gas.
- Biological processes, e.g., enzyme-catalyzed reactions.
Relating diagrams to everyday examples helps students make connections between theory and application, making the learning experience more tangible.
4. Interactive Elements to Engage Students

To foster an interactive learning environment, consider the following ideas:
- Worksheet Activities: Include fill-in-the-blank sections or questions that prompt students to analyze the diagram, like ‘What is the activation energy for this reaction?’
- Simulation Links: Provide links to interactive simulations where students can manipulate reactants and observe changes in the diagram.
- Drawing Exercises: Have students draw potential energy diagrams for simple reactions, fostering creativity and problem-solving skills.
Interactive elements not only increase engagement but also help in reinforcing the concepts through active participation.
5. Error Analysis and Troubleshooting

Understanding common mistakes in interpreting or drawing potential energy diagrams can provide clarity:
- Activation Energy Misinterpretation: Students might think it’s the energy change during the reaction rather than the energy needed to start the reaction.
- Energy Level Differences: Confusing the energy of reactants and products with activation energy.
- Exothermic vs. Endothermic Reactions: Not distinguishing between the two can lead to incorrect diagrams.
Incorporating these common errors into your worksheets can help students troubleshoot their understanding, leading to a deeper comprehension.
By considering these tips, educators can develop dynamic and educational worksheets that make potential energy diagrams not only a learning tool but also an engaging puzzle for students to solve. In crafting these materials, remember the importance of clear visuals, relatable examples, interactive components, and an understanding of common pitfalls. With this approach, you'll be fostering a generation of students equipped to visualize and comprehend complex energy changes in physical and chemical systems.
What is the main purpose of a potential energy diagram?

+
The main purpose of a potential energy diagram is to visually represent how the potential energy of a chemical system changes as a reaction proceeds, allowing for an understanding of energy changes, reaction feasibility, and activation energy.
How can I tell if a reaction is exothermic or endothermic from a diagram?

+
Exothermic reactions release energy, so the products will have lower potential energy than the reactants, leading to a downward curve. Conversely, endothermic reactions absorb energy, resulting in products with higher potential energy than reactants, with an upward curve.
Why is understanding the transition state crucial in these diagrams?

+
The transition state is the highest energy point on the curve, representing the moment of highest instability or highest energy requirement during the reaction. It’s crucial because it shows the energy barrier that must be overcome for the reaction to proceed, known as activation energy.



