5 Essential Tips for Mastering Planck's Constant Worksheet

Embarking on a journey through quantum physics can feel like navigating an intricate labyrinth, with concepts such as Planck's constant acting as the keys to unlocking the mysteries of the universe at its most fundamental level. For students and enthusiasts alike, mastering the calculation and application of Planck's constant through worksheets can be a pivotal step. Here are five essential tips to ensure you excel in this aspect of quantum mechanics:
Understand Planck’s Constant

Planck’s constant, denoted by the symbol h, is a physical constant that appears in equations describing the energy of particles at the quantum level. Its value is approximately (6.626 \times 10^{-34} ) joule-seconds. This constant quantifies the amount of energy in one quantum (or photon) of electromagnetic radiation, which is related to its frequency by the equation (E = hf).

💡 Note: Understanding what Planck’s constant represents is essential for grasping why it matters in quantum mechanics.
Master the Calculations

Worksheets often involve solving for energy, frequency, or wavelength of light using Planck’s constant. Here’s how to master these calculations:
- Learn the basic equations like (E = hf), where E is energy, h is Planck’s constant, and f is frequency.
- Relate wavelength (λ) to frequency with (c = f\lambda), where c is the speed of light.
- Practice conversion between units. For example, knowing (1 \, eV = 1.602 \times 10^{-19} \, J) can simplify calculations.
📚 Note: Regular practice with these equations will reduce errors and increase speed during exams.
Understand the Context

Planck’s constant isn’t just a number; it has context within physics:
- It’s fundamental to the quantum theory of energy quantization.
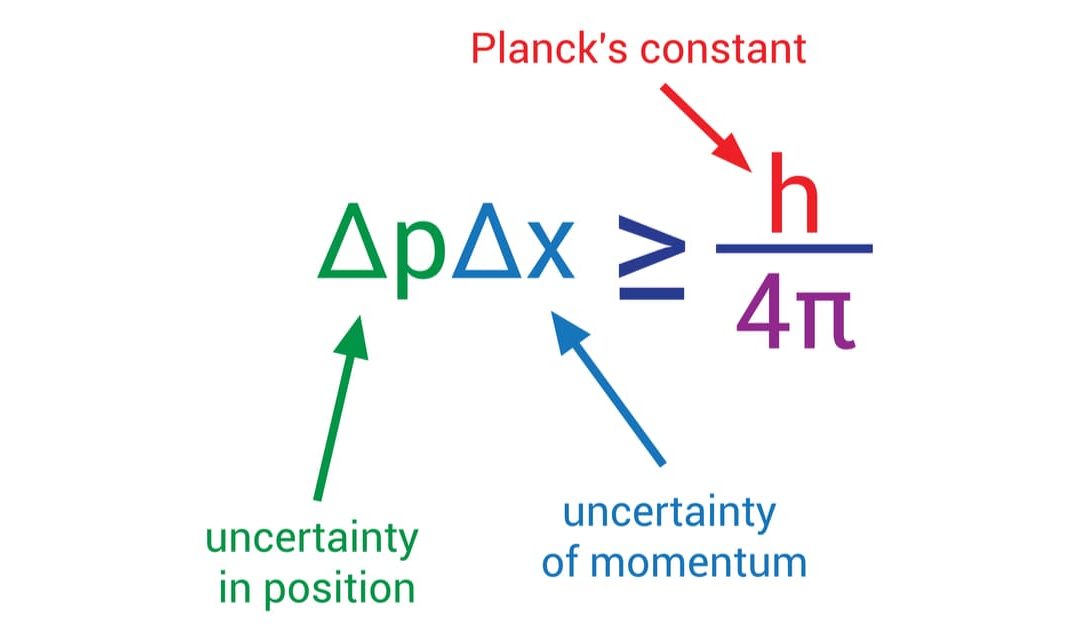
- It underpins the Heisenberg uncertainty principle, indicating the relationship between momentum and position uncertainties.
- It’s integral to understanding the photoelectric effect, where light energy is transferred to electrons.
🌟 Note: A deeper understanding of context will help you remember the applications and significance of Planck’s constant.
Work Through Problems Logically
Here’s a step-by-step process for approaching problems on Planck’s constant worksheets:
- Identify what you’re solving for (energy, frequency, etc.).
- Check if the units provided are consistent. If not, convert them to standard SI units.
- Apply the appropriate equation. If in doubt, use dimensional analysis to guide you.
- Plug in known values carefully, noting whether you need h or hbar (reduced Planck’s constant).
- Solve the equation, paying attention to significant figures and scientific notation.
🔍 Note: Understanding the logical flow of problem-solving can reduce common mistakes.
Seek Feedback and Collaboration

Learning doesn’t happen in isolation, especially in quantum physics:
- Join study groups or online forums where you can discuss problems and share insights.
- Engage with your professors or tutors for clarification on complex topics.
- Utilize resources like textbooks, online tutorials, and simulations to visualize concepts.
🤝 Note: Collaborative learning can expose you to different problem-solving strategies and deepen your understanding.
By applying these tips, you will not only master the calculations involving Planck's constant but also gain a deeper insight into the fascinating realm of quantum mechanics. As you work through your worksheets, remember that each problem is an opportunity to refine your understanding, not just to solve an equation. The ability to apply Planck's constant accurately will not only help in academic assessments but will also equip you with the knowledge necessary to comprehend and contribute to future scientific developments in fields like quantum computing, spectroscopy, and beyond.
Why is Planck’s constant important in quantum mechanics?

+
Planck’s constant quantifies the granularity of energy at the quantum level, meaning it sets the scale at which quantum effects become noticeable. It’s pivotal for concepts like the quantization of energy, wave-particle duality, and the uncertainty principle.
What is the reduced Planck’s constant (hbar)?

+
The reduced Planck’s constant, often denoted as hbar (ħ), is Planck’s constant divided by (2\pi). It’s used in quantum mechanics equations to simplify calculations, where angular frequency and rotational phenomena are involved.
Can I calculate Planck’s constant experimentally?

+
Yes, Planck’s constant can be determined experimentally through several methods, including the photoelectric effect experiment, where you measure the stopping potential for different frequencies of light to find the constant.
How does Planck’s constant relate to the photoelectric effect?

+
In the photoelectric effect, light energy is transferred to electrons, which are ejected from the surface of a material. Planck’s constant comes into play through Einstein’s equation: (KE{max} = hf - \phi), where (KE{max}) is the maximum kinetic energy of the electrons, (h) is Planck’s constant, (f) is the frequency of the incident light, and (\phi) is the work function of the material.
Is Planck’s constant relevant to everyday life?

+
While Planck’s constant describes phenomena at scales not directly observable in everyday life, its effects are indirectly influential. Quantum effects are critical in technologies like lasers, transistors, and MRI machines, which utilize the principles of quantum mechanics where Planck’s constant plays a fundamental role.