Physical vs. Chemical Properties: Interactive Worksheet Guide

The exploration of physical versus chemical properties forms a cornerstone of understanding in the realm of chemistry. For students, mastering these concepts can be both fun and enlightening, especially through interactive tools like worksheets. This comprehensive guide aims to elucidate the differences between physical and chemical properties, guide you through an interactive worksheet, and highlight how these principles apply in everyday life and advanced scientific study.
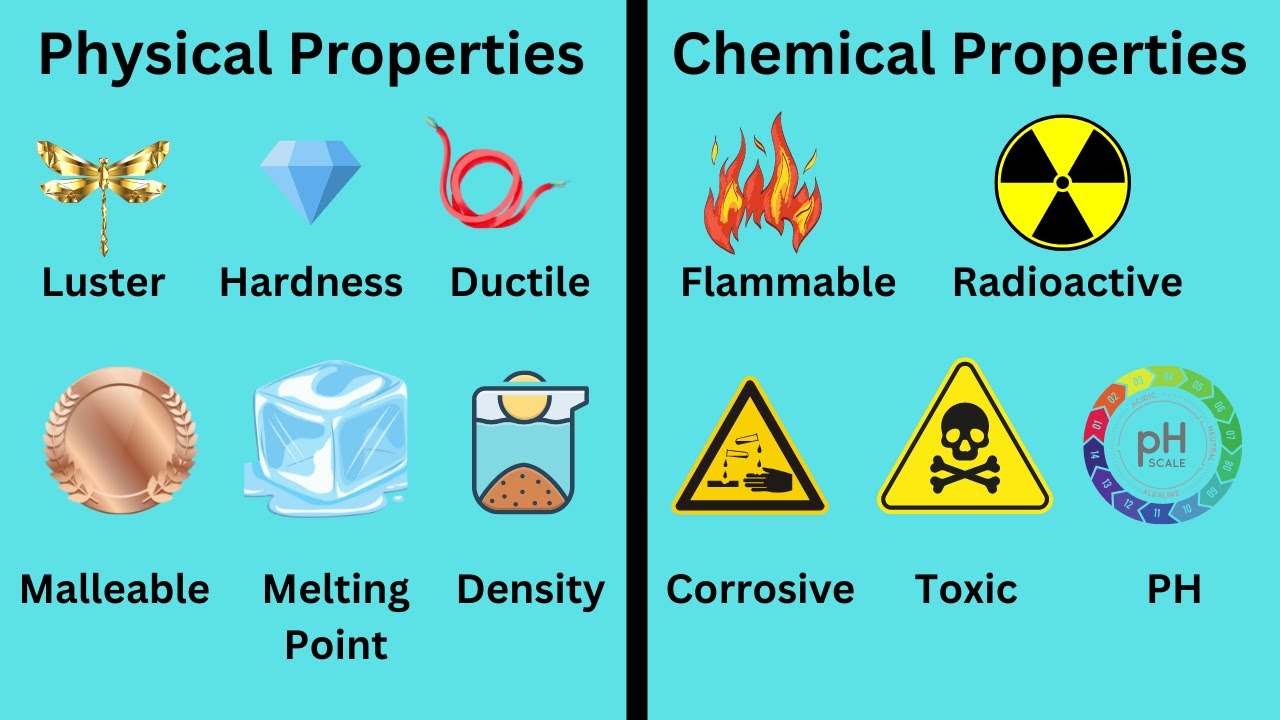
Understanding Physical Properties

Physical properties refer to characteristics of a substance that can be observed or measured without changing the chemical composition of the substance itself. Here are some key points:
- State of Matter: Whether a substance is a solid, liquid, or gas.
- Color: The hue or shade a substance exhibits.
- Density: Mass per unit volume, which can be used to identify substances.
- Boiling and Melting Points: Temperatures at which phase changes occur.
- Conductivity: Ability to conduct electricity or heat.
- Solubility: How well a substance dissolves in a solvent.
- Hardness: Resistance to physical deformation or scratching.

Understanding Chemical Properties

Chemical properties, on the other hand, involve the potential of a substance to undergo a chemical reaction to form new substances. Here's what you need to know:
- Flammability: The ability of a substance to burn or ignite.
- Reactivity: How readily a substance reacts with other substances.
- Toxicity: The capability to cause harmful effects to living organisms.
- Acidity or Basicity: Measured through pH, indicating the substance's behavior in an acid-base reaction.
- Oxidation States: The potential to gain or lose electrons in a reaction.

Interactive Worksheet Guide

An interactive worksheet can be an excellent tool to cement these concepts:
Step 1: Identifying Physical Properties
- Look at the provided samples or objects.
- List observable traits like color, shape, and state (solid, liquid, gas).
- Measure and record numerical values like mass, volume, and density.
Step 2: Experimenting with Chemical Properties

- Conduct simple experiments like burning a piece of paper or mixing vinegar with baking soda.
- Observe the changes in the substances (e.g., smoke, odor, temperature change, color change).
- Record these observations, noting that new substances are formed.
🔍 Note: Always ensure safe conditions and have proper adult supervision when conducting experiments involving chemical reactions.
Step 3: Comparing and Contrasting

- Fill out a comparison table on the worksheet with both physical and chemical properties for each substance.
| Substance | Physical Properties | Chemical Properties |
|---|---|---|
| Water | Colorless, liquid at room temp | Can react with acids or bases, supports combustion |
| Iron | Metallic gray, solid, conducts electricity | Oxidizes in air (rusts), reacts with acids |

🔎 Note: The given table should be expanded to include more substances for better understanding and practice.
Everyday Applications

Understanding physical and chemical properties is not just for the laboratory; it has real-world implications:
- Cooking: The physical properties of ingredients like melting point affect how we cook (e.g., melting butter to make sauce).
- Materials Science: Engineers use properties to select appropriate materials for construction or manufacturing.
- Environmental Impact: Chemical properties like toxicity affect how substances interact with the environment.
Advanced Scientific Study

For those delving deeper into science:
- Analytical Chemistry: Techniques like spectroscopy analyze physical and chemical properties.
- Forensic Science: Identifying substances through their properties is crucial in investigations.
- Pharmaceutical Development: The solubility and reactivity of drugs are studied to optimize delivery methods.
Through the use of interactive worksheets, students can actively engage with these concepts, making learning more impactful and enjoyable. This hands-on approach not only aids in memorizing facts but also in understanding the underlying principles that govern the behavior of matter.
What’s the difference between physical and chemical changes?

+
Physical changes affect the form of a chemical substance but not its chemical identity, like melting ice into water. Chemical changes, on the other hand, produce new substances with new properties, such as rusting of iron.
Can a physical property change during a chemical reaction?

+
Yes, physical properties can change during a chemical reaction as new substances are formed, which will have their own unique physical characteristics.
How can I easily remember the difference between physical and chemical properties?

+
A simple mnemonic could be “Physical Properties: Perceived Without a Reaction; Chemical Properties: Change Composition.” Physical properties can be observed without altering the substance’s identity.



