Mastering Chemical Reactions: Your Essential Worksheet Guide

Chemical reactions are the cornerstone of chemistry, and understanding them is crucial for both academic success and real-world applications. Whether you're a student preparing for an exam or a chemistry enthusiast eager to expand your knowledge, mastering chemical reactions can significantly enhance your comprehension of how the world works at the molecular level. This guide aims to provide you with an extensive worksheet designed to help you excel in this fundamental area of chemistry.
Understanding Chemical Reactions
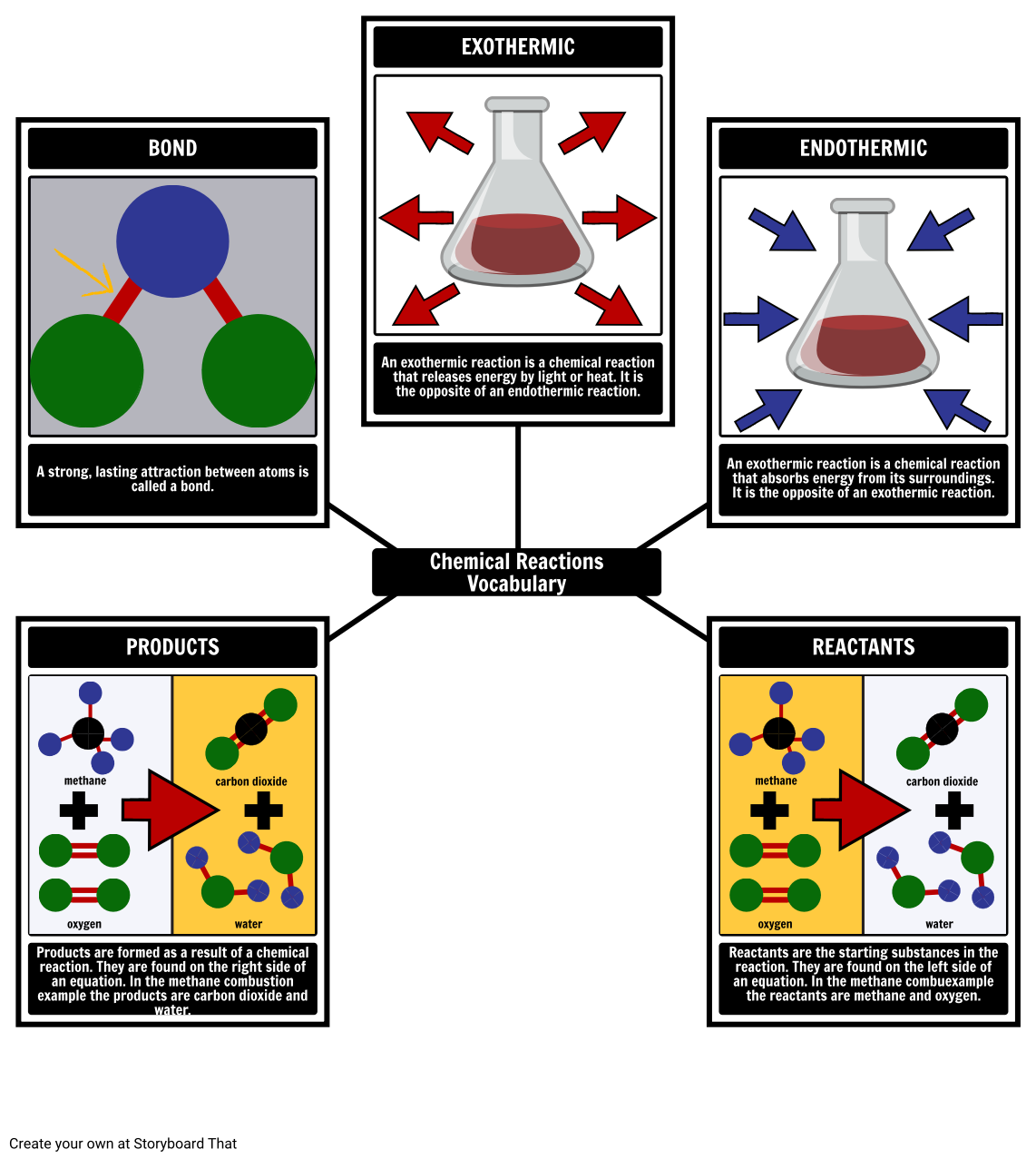
Before diving into the worksheet, let's clarify what a chemical reaction is:
- Definition: A chemical reaction is a process that transforms one or more substances, called reactants, into different substances known as products through the breaking and forming of chemical bonds.
- Types of Reactions: Reactions can be classified into several categories like synthesis, decomposition, single displacement, double displacement, combustion, and neutralization.
- Signs of a Chemical Reaction: Change in temperature, color change, gas formation, and the appearance of a precipitate are typical signs that a chemical reaction has occurred.
Worksheet Components

The worksheet provided below will cover various aspects of chemical reactions:
- Nomenclature: Understanding and naming chemical compounds correctly.
- Balancing Equations: Balancing chemical equations to conserve mass and atoms.
- Identifying Reaction Types: Categorizing reactions based on their characteristics.
- Predicting Products: Forecasting the products from given reactants.
- Calculations: Performing stoichiometry calculations, including mass-mass, mole-mole, and limiting reactant scenarios.
Nomenclature Practice

Let's start with nomenclature:
| Compound | Name |
|---|---|
| NaCl | Sodium Chloride |
| Fe₂O₃ | Iron(III) Oxide |
| H₂SO₄ | Sulfuric Acid |

Balancing Equations

Balancing equations is critical for understanding the conservation of matter:
- Na + Cl₂ → NaCl
- C + O₂ → CO₂
- Al + HCl → AlCl₃ + H₂
Complete each equation by balancing the atoms on both sides:
📝 Note: Remember that hydrogen and oxygen usually balance last, and check each equation twice for accuracy.
Identifying Reaction Types

Recognize the type of reaction from the following:
- Mg + O₂ → MgO (Synthesis)
- HCl + NaOH → NaCl + H₂O (Neutralization)
- CaCO₃ → CaO + CO₂ (Decomposition)
Predicting Products

Given the reactants, predict the products:
- AgNO₃(aq) + NaCl(aq) →
💡 Note: This is a precipitation reaction, so you'll form AgCl(s) and NaNO₃(aq).
- KOH(aq) + H₂SO₄(aq) →
💡 Note: This is an acid-base reaction, resulting in K₂SO₄(aq) and H₂O(l).
Stoichiometry Calculations

Practice stoichiometry problems to understand the quantitative relationships between reactants and products:
- Calculate the mass of CO₂ produced when 50g of methane (CH₄) burns completely with excess oxygen.
- Determine the limiting reactant when 2.5 moles of N₂ react with 6 moles of H₂ to form ammonia (NH₃).
Summing Up

Throughout this guide, we've delved into the fundamental aspects of chemical reactions, providing a comprehensive worksheet to sharpen your skills. From understanding the types of reactions to practicing stoichiometry calculations, these exercises are designed to solidify your grasp on how substances interact at the atomic level. Remember, mastering chemical reactions isn't just about memorizing formulas or reaction types; it's about understanding the underlying principles of matter transformation, the conservation laws, and how these reactions are integral to our daily lives.
What are the common types of chemical reactions?

+
The most common types include synthesis, decomposition, single displacement, double displacement, combustion, and neutralization.
Why is it important to balance chemical equations?

+
Balancing equations is key to ensure that the law of conservation of mass is upheld, showing that the total mass of reactants equals the total mass of products.
Can I predict reaction products without memorizing every possibility?

+
Yes, by understanding the reactivity series and the fundamental principles of reaction types, you can often predict products based on patterns and rules.
What’s the significance of reaction stoichiometry?

+
Reaction stoichiometry helps determine the quantitative relationship between reactants and products, crucial for manufacturing, environmental science, and biochemistry.