5 Proven Answers for Phase Changes Worksheet Success

When it comes to understanding phase changes in chemistry or physics, a phase changes worksheet can be both a tool for learning and a challenge. Phase changes or state changes occur when a substance transforms from one state of matter to another, like from solid to liquid or from liquid to gas. These transformations are governed by changes in temperature, pressure, or sometimes both. This blog post will guide you through five proven answers that ensure you ace every phase change worksheet you encounter, with insights tailored for seamless understanding and application.
Understanding the Basics of Phase Changes
Phase changes are a crucial aspect of physical science because they illustrate the dynamic behavior of matter under different conditions. Here’s what you need to know:
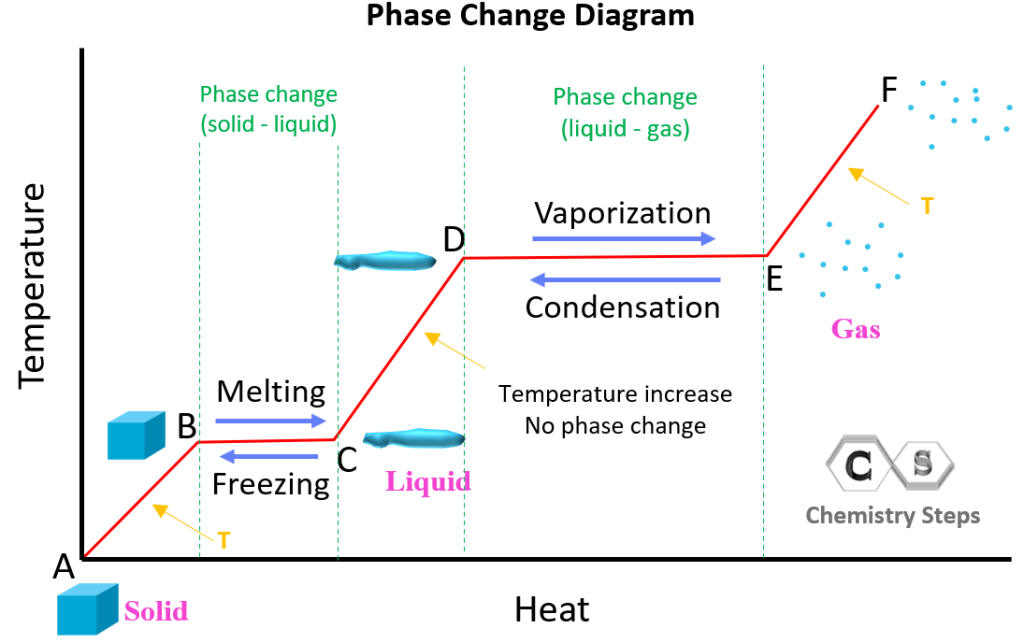
- Melting and Freezing: These are opposite processes. Melting is when a solid turns into a liquid, usually by increasing the temperature, while freezing happens when a liquid turns into a solid by decreasing the temperature.
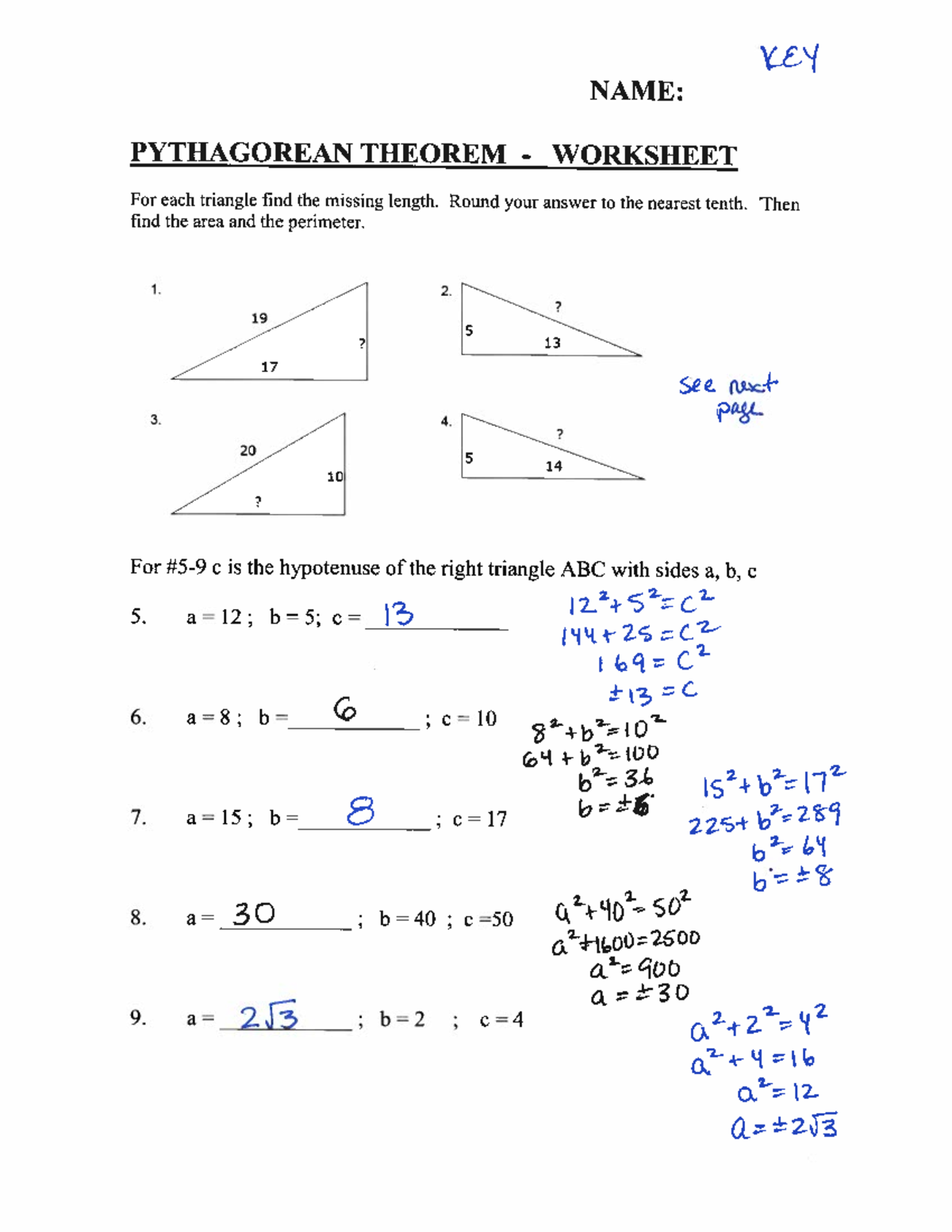
- Boiling and Condensation: Boiling, or vaporization, occurs when a liquid turns into a gas, which involves adding heat. Conversely, condensation is when a gas turns into a liquid, often seen when steam meets a colder surface.
- Sublimation and Deposition: Sublimation is when a solid directly turns into a gas without passing through the liquid phase, like dry ice sublimating. Deposition is the reverse process, where a gas turns directly into a solid.
- Evaporation: This is similar to boiling but occurs at a temperature below the boiling point, often at the surface of a liquid.
📝 Note: All these processes can be explained by the concept of energy transfer, which either breaks or forms bonds between particles.
Analyzing Common Phase Changes Worksheet Questions
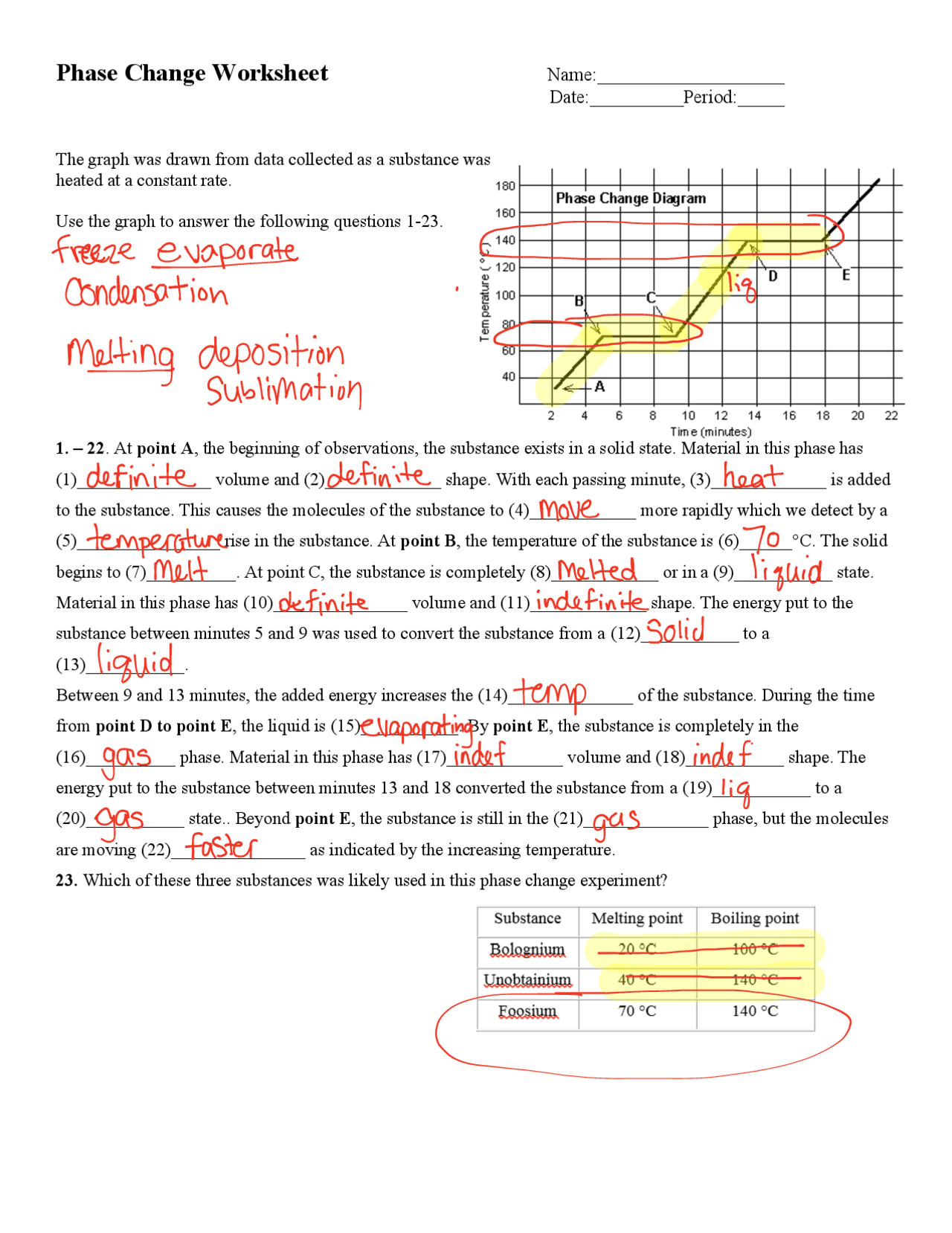
Worksheet questions typically focus on:
- Defining key terms: What is the difference between melting and boiling?
- Thermodynamic considerations: How does the heat of fusion relate to melting?
- Practical Applications: What phase changes occur in refrigeration?
- Energy exchange: How much energy is required to turn ice into steam?
| Phase Change | Change of State | Energy Change | Example |
|---|---|---|---|
| Melting | Solid to Liquid | Absorption of Heat | Ice turning into water |
| Freezing | Liquid to Solid | Release of Heat | Water freezing into ice |
| Boiling | Liquid to Gas | Absorption of Heat | Water boiling into steam |
| Condensation | Gas to Liquid | Release of Heat | Steam condensing back to water |
| Sublimation | Solid to Gas | Absorption of Heat | Dry ice turning into CO2 gas |

📚 Note: Understanding the energy flow during each phase change is crucial for mastering worksheets and practical applications in physics and chemistry.
Mastering Key Formulas for Phase Changes

Here are some fundamental formulas you’ll need to conquer any phase changes worksheet:
- Heat of Fusion (Hfus): Q = m × Hfus where Q is the heat energy, m is the mass of the substance, and Hfus is the latent heat of fusion.
- Heat of Vaporization (Hvap): Q = m × Hvap similar to Hfus, but for boiling or evaporation.
- Change in Temperature: Q = m × c × ΔT where Q is the heat energy, m is mass, c is specific heat capacity, and ΔT is the change in temperature.
These formulas help in calculating the amount of heat absorbed or released during phase changes. Apply them accurately to ensure your worksheet solutions are correct.
💡 Note: Always check units when solving problems involving phase changes; mass in grams, energy in joules, temperature in degrees Celsius or Kelvin, depending on the given data.
Common Mistakes in Phase Changes Worksheets and How to Avoid Them
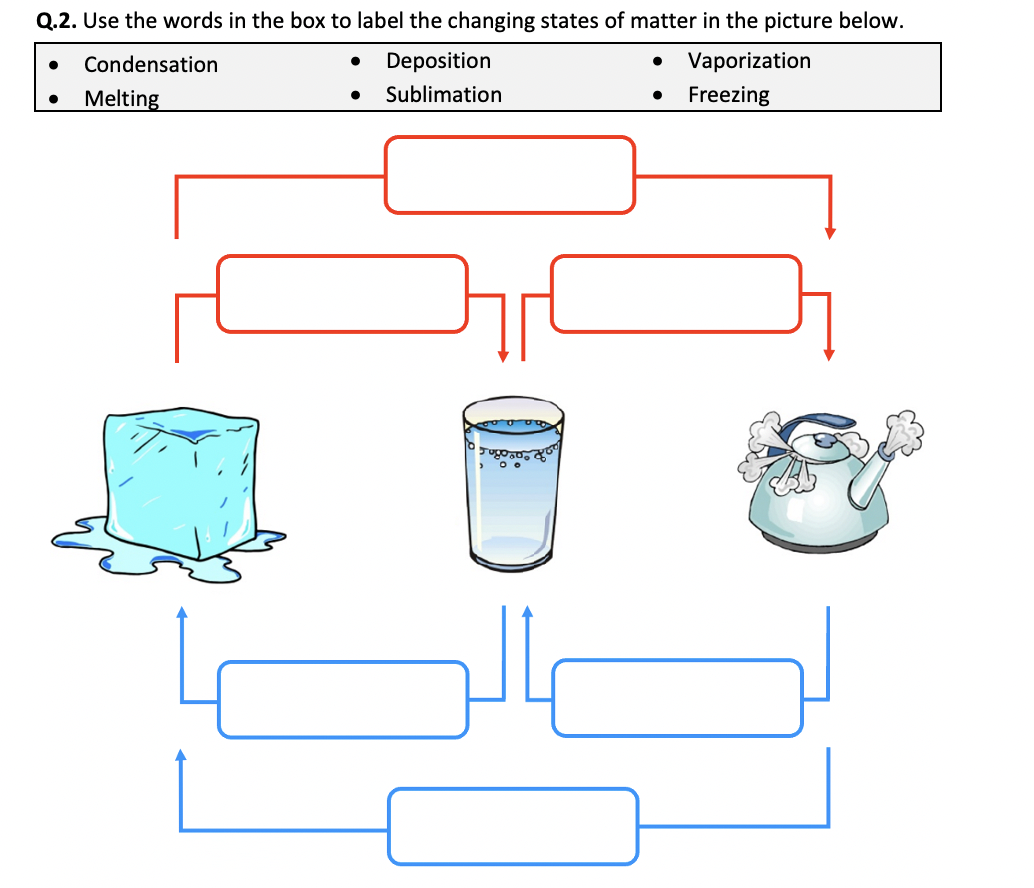
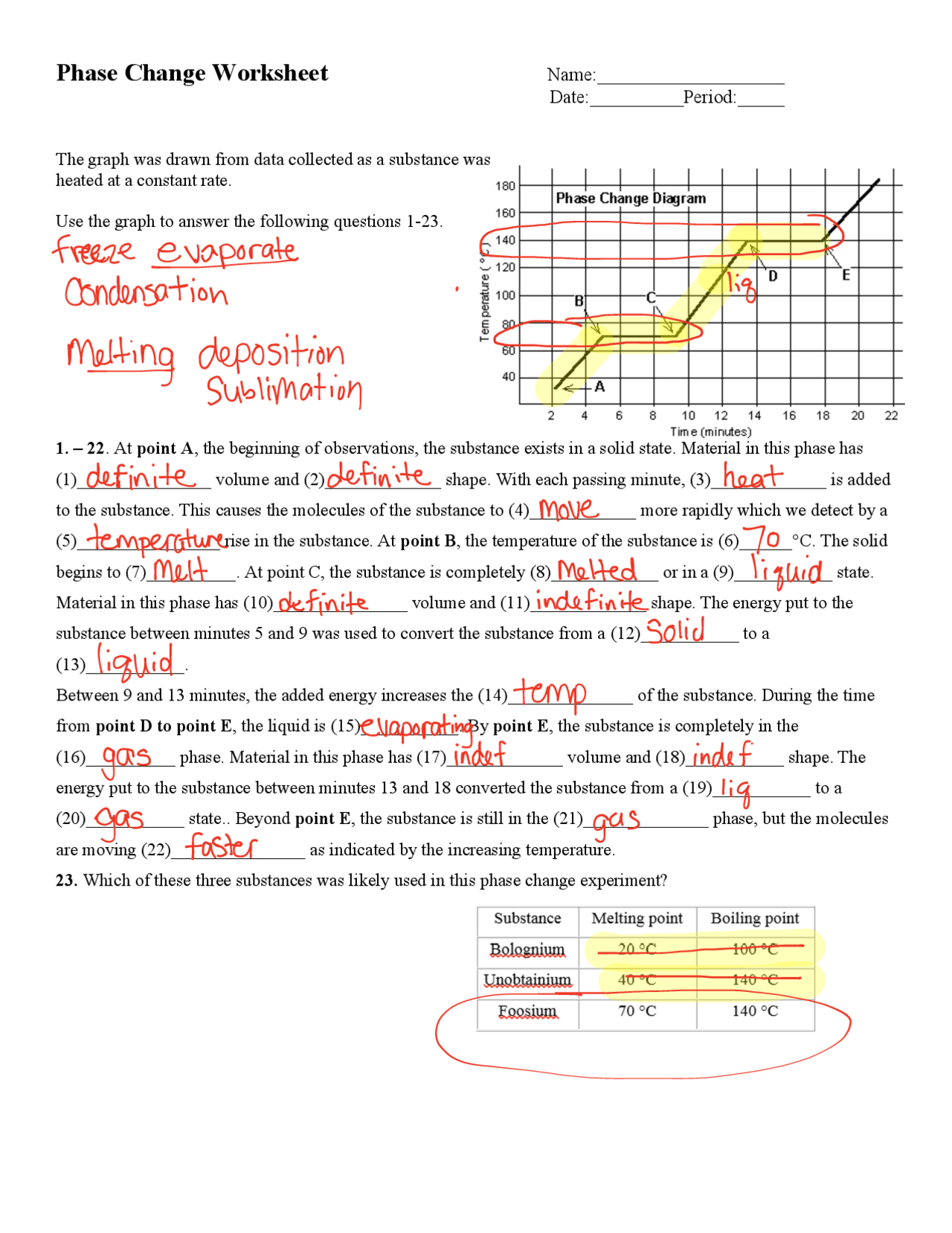
Students often make several mistakes when dealing with phase changes:
- Confusing Heat Energy with Temperature: Heat is energy transfer, and temperature is a measure of the average kinetic energy of particles. Always ensure you’re solving for the right variable.
- Ignoring Latent Heat: Latent heat does not increase the temperature but changes the state of the matter. Remember to include it in calculations.
- Units Mismatching: Keep units consistent throughout calculations to avoid conversion errors.
- Incorrect Phase Identification: Accurately identify the phase of matter at each step in the problem. A common error is assuming that a substance is at its boiling or freezing point without proper context.
🔍 Note: Always double-check the phase diagram provided in the worksheet or your understanding of the substance's behavior at different temperatures and pressures.
Strategies for High Scores on Phase Changes Worksheets

To excel in these worksheets:
- Read Thoroughly: Understand each question completely before attempting to solve it.
- Organize Your Work: Break down each step of the problem, showing calculations for each phase change or temperature change.
- Check Your Units: Make sure units match throughout your work. Convert if necessary.
- Consider Real-World Examples: Think of real-life scenarios where phase changes occur to solidify your understanding.
- Review Material: Go over phase changes, their associated energies, and thermodynamics principles regularly.
In the final analysis, mastering phase changes is not just about memorizing formulas or terms; it’s about understanding how matter responds to energy changes. This knowledge is fundamental for both theoretical understanding and practical applications in numerous fields, from industrial processes to everyday life situations like cooking or weather phenomena.
What is the difference between latent heat of fusion and latent heat of vaporization?
+
The latent heat of fusion is the amount of heat required to change a solid into a liquid at its melting point, while the latent heat of vaporization is the amount of heat required to convert a liquid into a gas at its boiling point. The key difference lies in the phases being changed: from solid to liquid (fusion) or liquid to gas (vaporization).
Can phase changes occur without a change in temperature?

+
Yes, during phase changes, the substance absorbs or releases latent heat, which does not cause a temperature change. Instead, the energy is used to break or form intermolecular bonds. For example, ice melts at 0°C without changing in temperature until all the ice has turned into water.
How does pressure affect phase changes?

+
Pressure can significantly affect phase changes. Increasing pressure raises the boiling point of a liquid and can prevent boiling altogether by increasing the pressure on the liquid’s surface. Conversely, decreasing pressure can lower the freezing point of a substance, like in the case of vacuum freezing or freeze-drying processes.