5 Essential Tips for Mastering pH and pOH Calculations

Understanding pH and pOH Fundamentals

Before we delve into the intricacies of pH and pOH calculations, let's briefly review the basics. The pH scale measures the acidity or alkalinity of a solution, ranging from 0 to 14. Solutions with a pH below 7 are considered acidic, while those above 7 are basic, and 7 is neutral. pOH, on the other hand, measures the alkalinity or the concentration of hydroxide ions (OH-) in a solution. It's the logarithmic expression of the concentration of OH- ions, calculated as the negative logarithm (base 10) of the OH- ion concentration. Here's a key fact to remember:
- pH + pOH = 14
This equation shows the relationship between pH and pOH in an aqueous solution at 25°C.
Tip #1: Master the Logarithms

Understanding logarithmic calculations is crucial for pH and pOH because they involve logarithmic scales. Here are some pointers:
- The pH is calculated as pH = -log[H+], where [H+] is the concentration of hydrogen ions in moles per liter.
- Similarly, pOH is calculated as pOH = -log[OH-], where [OH-] is the concentration of hydroxide ions.
- The logarithm property, loga(b) = c where ac = b, is essential. For example, if [H+] = 1 x 10-3 M, then pH = -log(1 x 10-3) = 3.
💡 Note: Logarithmic functions are not linear, meaning changes in pH reflect exponential changes in hydrogen ion concentration.
Tip #2: Use the Inverse Log Function
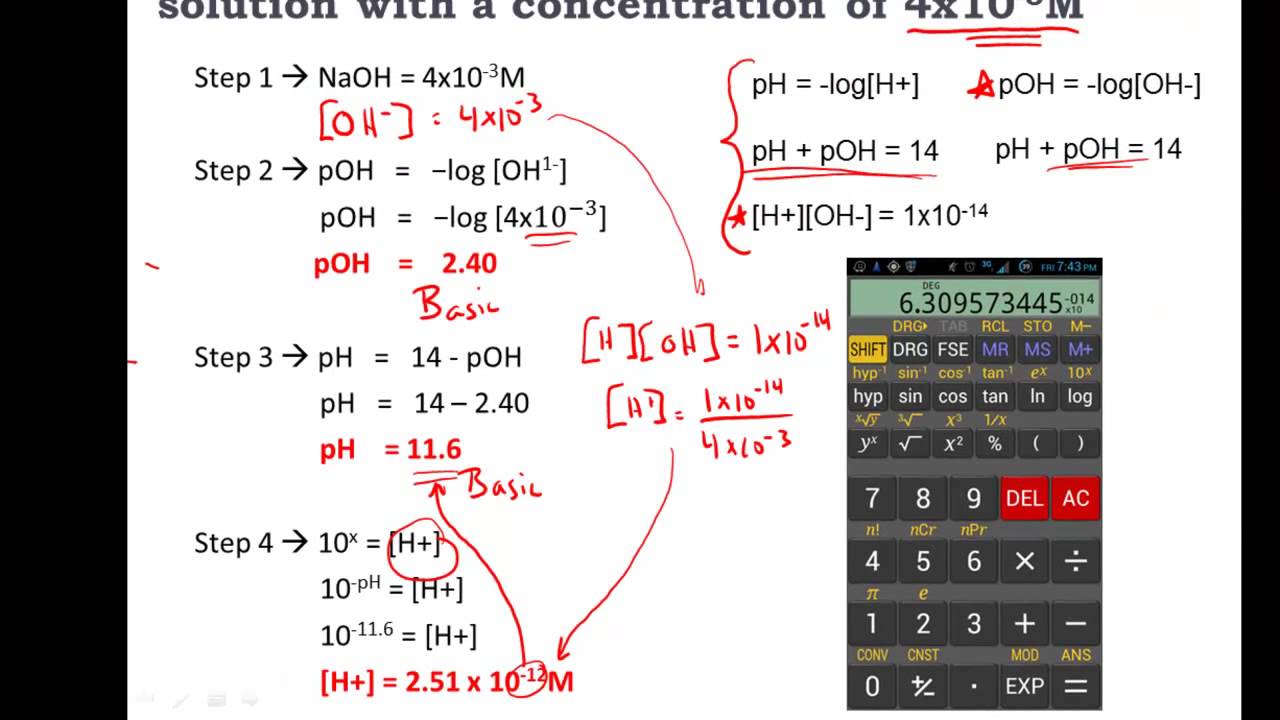
Calculating [H+] or [OH-] from given pH or pOH values requires the inverse logarithm, also known as the antilog. Here's how to approach this:
- If the pH of a solution is 5, to find [H+], you calculate [H+] = 10-pH = 10-5 = 1 x 10-5 M.
- Similarly, for pOH, if pOH is 9, then [OH-] = 10-pOH = 10-9 = 1 x 10-9 M.
Tip #3: Understand Solution Concentrations

Accurate pH and pOH calculations require an understanding of solution concentrations. Here are some key points:
- Molarity (M) is the concentration of solute per liter of solution. It's expressed as moles of solute per liter of solution.
- Dilution and concentration changes affect pH and pOH. If a solution is diluted, its pH will increase (become more basic) because the concentration of [H+] is reduced.
- When calculating pH and pOH, always consider the total volume of the solution after all additions or reactions.
| Change in Concentration | Effect on pH |
|---|---|
| Dilution | Becomes more basic (pH increases) |
| Concentration | Becomes more acidic (pH decreases) |

⚠️ Note: Remember that diluting a solution does not alter the pOH in the same way because pOH measures OH- ion concentration, which remains constant in an aqueous solution.
Tip #4: Account for Temperature Effects

Temperature influences the dissociation of water (Kw), affecting pH and pOH. Here's what you need to know:
- At 25°C, Kw = 1 x 10-14, which means pH + pOH = 14.
- At other temperatures, this product changes. As temperature increases, water dissociates more, leading to a higher concentration of both H+ and OH- ions, altering the pH and pOH values.
Tip #5: Practice with Calculations and Buffer Systems

Lastly, the only way to become proficient in pH and pOH calculations is through practice. Here's how:
- Work on sample problems to understand how pH and pOH interact in different scenarios.
- Bufffer systems maintain pH by responding to the addition of acids or bases. Understanding buffer systems like buffer solutions and Henderson-Hasselbalch equation helps in mastering pH control.
Mastering pH and pOH calculations opens up a world of understanding in chemistry, from predicting the outcomes of chemical reactions to managing environmental systems like water treatment. By mastering logarithms, understanding inverse functions, considering concentrations, accounting for temperature, and practicing with buffer systems, you can become an adept in managing pH and pOH values in any lab or field setting.
The journey through pH and pOH is continuous learning, requiring patience and practice. As you navigate through various scenarios, remember these tips, and you'll find your calculations becoming more accurate and your understanding deepening.
What is the difference between pH and pOH?

+
The pH scale measures the concentration of hydrogen ions (H+) in a solution, indicating acidity or alkalinity. The pOH scale measures the concentration of hydroxide ions (OH-) in the solution. The sum of pH and pOH equals 14 in an aqueous solution at 25°C.
How does temperature affect pH and pOH calculations?

+
Temperature changes the dissociation constant of water (Kw), which affects the pH and pOH because it alters the concentration of hydrogen and hydroxide ions in the solution.
Why is mastering logarithms important for pH and pOH calculations?

+
Logarithms are fundamental to pH and pOH because these scales are logarithmic expressions of ion concentration. Understanding logarithms enables you to interpret and calculate these values accurately.
What role do buffer systems play in pH management?

+
Buffer systems resist changes in pH by either absorbing or releasing hydrogen ions when acids or bases are added. They are crucial for maintaining a stable pH in biological systems and chemical processes.



