5 Tips for Naming Covalent Compounds Easily

5 Tips for Naming Covalent Compounds Easily

Understanding how to name covalent compounds is essential for students of chemistry. Covalent compounds are formed when two non-metal atoms bond together by sharing electrons. Here are five tips to help you master this aspect of chemistry:
1. Use Prefixes to Indicate the Number of Atoms

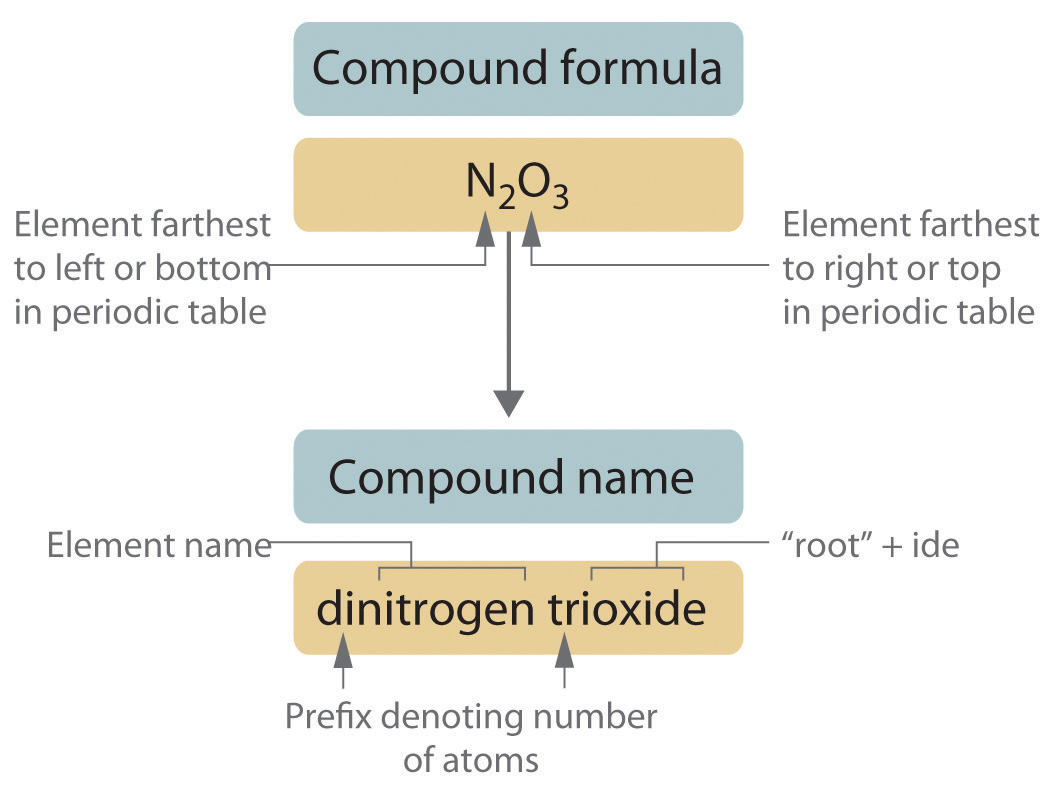
Covalent compounds utilize numerical prefixes to denote the number of atoms present in each element within the compound. Here's a list of common prefixes:
- mono-: 1
- di-: 2
- tri-: 3
- tetra-: 4
- penta-: 5
- hexa-: 6
- hepta-: 7
- octa-: 8
- nona-: 9
- deca-: 10
For instance, when you see the formula P2O5, you name it by using the prefix 'di' for two Phosphorus atoms and 'penta' for five Oxygen atoms, thus it is diphosphorus pentoxide.
📝 Note: The prefix 'mono' is generally omitted for the first element in the name, except when needed for clarity.
2. Name the Elements in Order

Start by naming the less electronegative element first, followed by the more electronegative one. Here's how it works:
- Phosphorus (P) is less electronegative than Oxygen (O), so P2O5 becomes diphosphorus pentoxide.
- Carbon (C) is less electronegative than Nitrogen (N), so CN becomes carbon nitride, but since it's a binary molecular compound, you'd name it carbon monoxide if it had only one oxygen, or nitrogen would be the first named in the formula (like in NO).
🧪 Note: Electronegativity can be confusing; however, a basic guideline is that elements closer to fluorine are generally more electronegative.
3. Change the Ending to '-ide'

The second element in the formula always ends in '-ide' to indicate a compound. For example:
- CCl4 is carbon tetrachloride, where 'chloride' is derived from chlorine.
- NH3 is nitrogen trihydride, though it's commonly known as ammonia.
Even if the elements are not in their standard forms, the '-ide' ending remains consistent.
4. Mind the Exceptions

Some compounds, due to their historical significance or common usage, have traditional names:
- H2O - Water (instead of hydrogen oxide)
- NO - Nitric oxide (instead of nitrogen monoxide)
- N2O - Nitrous oxide or laughing gas (instead of dinitrogen monoxide)
These names are so entrenched in scientific literature that they are universally recognized and used. Learning these exceptions can be as important as learning the rules.
5. Use Tables for Complex Compounds
To help memorize or understand complex covalent compounds, tables can be immensely useful. Here is a simple example:
| Formula | Name | Number of atoms |
|---|---|---|
| PCl3 | Phosphorus trichloride | 1 P, 3 Cl |
| SO2 | Sulfur dioxide | 1 S, 2 O |
| N2O4 | Dinitrogen tetroxide | 2 N, 4 O |

Creating or referencing such tables can aid in identifying patterns and committing them to memory.
Summing Up the Keys to Naming Covalent Compounds

Naming covalent compounds correctly involves understanding the structure of the compound, recognizing prefixes, ordering elements, and knowing when to change suffixes and memorize exceptions. With these tips in mind, you can simplify what might seem like a complex task. Practice using common compounds as examples, and over time, the naming of covalent compounds will become second nature.
Why do covalent compounds use prefixes?

+
Prefixes indicate the number of atoms of each element in the compound, helping to distinguish between different compounds formed by the same elements.
When do we use the ‘mono’ prefix?

+
The ‘mono’ prefix is used for the second element in the compound if there’s only one atom of that element, but it’s typically omitted for the first element, except in cases where clarity is needed or in more complex compounds.
Why don’t some common compounds follow the naming rules?

+
Some compounds have traditional or common names established long before systematic naming conventions were widely adopted, leading to the retention of these names due to historical and practical reasons.



