5 Tips for Mastering Molecular Shapes in Chemistry

Understanding molecular shapes is crucial for students and enthusiasts of chemistry, as it forms the foundation of various chemical concepts including polarity, reactivity, and bonding. Here are five detailed tips to help you master the intricate world of molecular geometries:
1. Master the Concept of Electron Domains

Before diving into shapes, ensure you understand how electrons are organized in molecules. Electron domains refer to regions around an atom where electrons, either in bonds or as lone pairs, are located. Here’s how to understand this:
- Each bond (single, double, or triple) counts as one domain.
- Each lone pair counts as one domain.
- Using the VSEPR (Valence Shell Electron Pair Repulsion) theory, you can predict the shape of molecules by noting that electron domains repel one another.
🔍 Note: A double or triple bond will take up more space than a single bond due to electron repulsion, affecting the final geometry.
2. Use VSEPR Theory to Determine Molecular Geometry
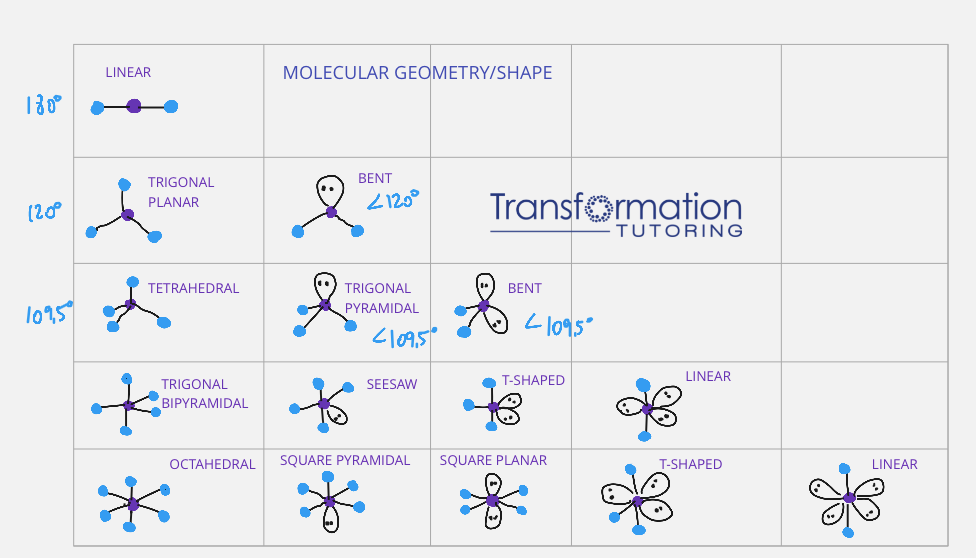
VSEPR theory is your roadmap to molecular shapes. Here's a basic rundown:
| Number of Electron Domains | Molecular Geometry |
|---|---|
| 2 | Linear |
| 3 | Trigonal Planar |
| 4 | Tetrahedral |
| 5 | Trigonal Bipyramidal |
| 6 | Octahedral |
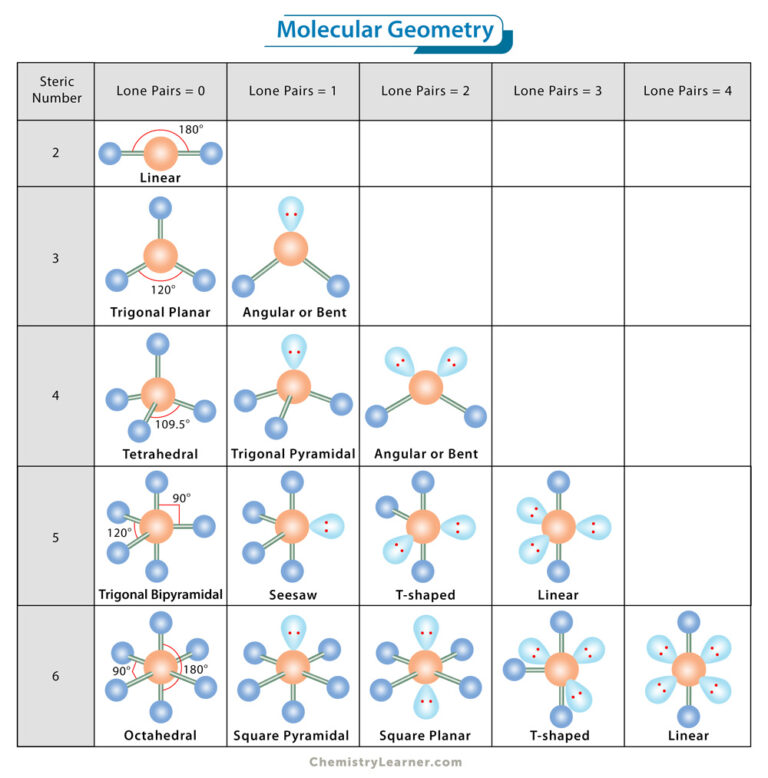
By counting electron domains around the central atom, you can predict the basic shape of the molecule. Remember, lone pairs occupy space but they don’t contribute to the molecular shape.
3. Account for Lone Pairs and Bond Angles

Lone pairs on central atoms can alter bond angles due to increased repulsion compared to bonded pairs. Here’s what you need to know:
- Lone pair-lone pair repulsion > lone pair-bonded pair repulsion > bonded pair-bonded pair repulsion.
- Bond angles decrease as the number of lone pairs increases. For example:
- With no lone pairs, methane (CH4) has bond angles of 109.5°.
- With one lone pair, ammonia (NH3) has bond angles around 107°.
🚨 Note: Lone pairs push bonded pairs closer together, which can dramatically change the molecule's shape and reactivity.
4. Memorize Common Molecular Shapes

While VSEPR theory helps predict molecular geometry, knowing common shapes saves time. Here are a few:
- Linear: Molecules like BeCl2 or CO2.
- Bent: H2O or SO2, where lone pairs push the bonded atoms closer.
- Trigonal Planar: BF3 or any molecule with three bonds and no lone pairs on the central atom.
- Tetrahedral: CH4, NH4+.
- Pentagonal Bipyramidal: ICl5 or SF5-.
5. Apply Your Knowledge to Understand Polarity

Molecular geometry directly influences a molecule’s polarity, which is important for understanding properties like solubility, reactivity, and boiling points. Here's how:
- If a molecule has a symmetrical shape and all surrounding atoms are identical, it's non-polar (e.g., CO2).
- An asymmetrical arrangement can lead to a polar molecule if different atoms have different electronegativities (e.g., H2O).
Mastering molecular shapes not only enhances your understanding of chemical bonding but also helps in grasping the physical and chemical properties of compounds. Chemistry becomes fascinating when you can visualize these invisible structures, predict behaviors, and solve chemical puzzles. This knowledge equips you to tackle more complex topics like coordination complexes, crystal structures, and the fascinating world of macromolecules. Remember, the key to mastering chemistry is not just memorization but understanding the underlying principles that govern molecular behaviors.
What is the VSEPR theory?

+
VSEPR stands for Valence Shell Electron Pair Repulsion theory. It states that electron pairs, whether bonding or non-bonding, repel each other, causing the atoms in a molecule to be positioned as far apart as possible, thereby determining the molecular shape.
How does the presence of lone pairs affect molecular geometry?

+
Lone pairs take up more space than bonded pairs due to greater repulsion, leading to a reduction in bond angles and altering the molecule’s shape.
What are some common molecular shapes?

+
Common shapes include linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral, bent, T-shaped, and see-saw, among others.
Why is understanding molecular geometry important?

+
Understanding molecular geometry is crucial for predicting molecule behavior, including reactivity, polarity, hybridization, and even solubility and boiling points.
Can a molecule with non-polar bonds be polar?

+
Yes, if the molecular geometry results in an asymmetrical distribution of charge, even with non-polar bonds, the molecule can exhibit net polarity.