5 Mole Conversion Tips You Need Now

In the realm of chemistry, understanding mole conversions is vital. Whether you're a student grappling with stoichiometry, or a professional dealing with chemical synthesis, mastering these conversions is crucial. This post will cover five essential mole conversion tips that will enhance your proficiency and understanding of this fundamental concept.
Understanding the Mole Concept

The mole concept is foundational in chemistry. It acts as a bridge between macroscopic measurements and the atomic or molecular scale. Here’s what you need to understand:
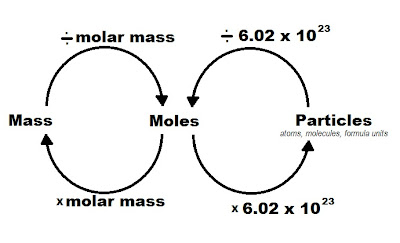
- Avogadro’s Number: 6.022 x 1023 particles per mole.
- Molar Mass: The mass of one mole of a substance, which can be found by summing the atomic masses of its elements.
- Mole-Mole Conversions: Using balanced chemical equations to determine the mole ratios between reactants and products.
Tip 1: Use Dimensional Analysis
Dimensional analysis, or the factor-label method, is an effective tool for mole conversions. Here’s how it works:
- Start with the given quantity and label its units.
- Use conversion factors that cancel out the initial units, leading to the desired unit. For example:
- 1 mole of substance = 6.022 x 1023 particles
- Molar mass (grams per mole) for mass to moles or vice versa
- Chemical formula or equation for mole-to-mole conversions
Let’s illustrate this with an example:
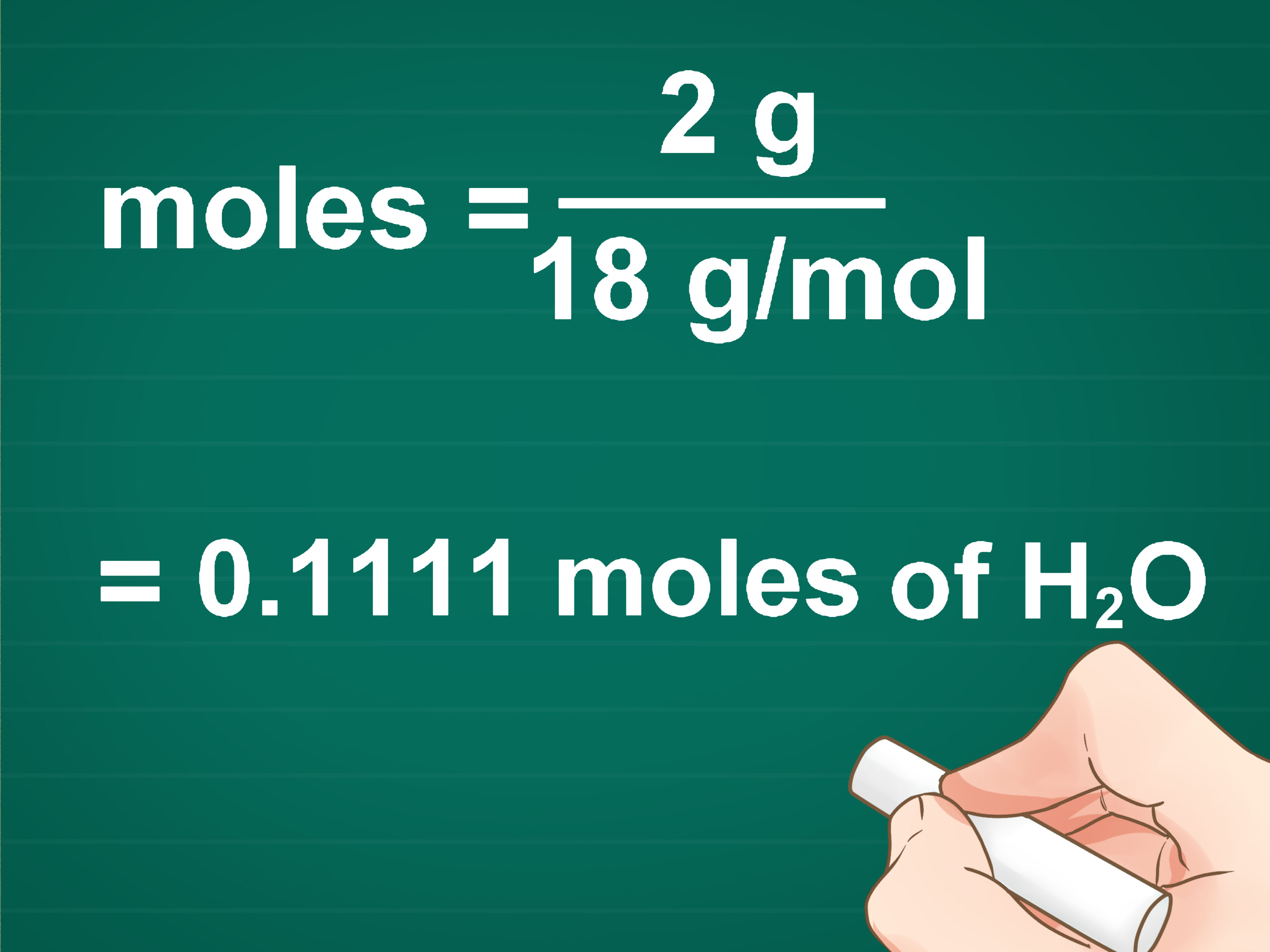
How many moles are in 5.8 grams of sodium chloride (NaCl)?
- 5.8 g NaCl * (1 mol NaCl / 58.44 g NaCl) = 0.0993 mol NaCl
✏️ Note: Always ensure your units cancel out in the numerator and denominator to arrive at the correct unit of measure.
Tip 2: Master the Use of Stoichiometry

Stoichiometry involves using the balanced chemical equation to determine the amount of reactants or products in a chemical reaction:
- Identify the reactants and products.
- Determine the mole ratio from the coefficients in the balanced equation.
- Calculate the amount of one substance given the amount of another using this ratio.
Example:
If you burn 2 moles of hydrogen gas (H2) to produce water (H2O), how many moles of water will you get?
- 2H2(g) + O2(g) → 2H2O(l)
- Given the ratio is 2:2 or 1:1, you’ll get 2 moles of H2O.
Tip 3: Practice With Molarity
Molarity (M) is defined as moles of solute per liter of solution:
Molarity = moles of solute / volume of solution (in liters)
Use this formula to:
- Convert from volume to moles or vice versa.
- Understand concentration in a solution-based reaction.
For example:
If you have 2.5 liters of a 0.2M HCl solution, how many moles of HCl are present?
- 0.2 mol/L * 2.5 L = 0.5 moles of HCl
Tip 4: Utilize Gas Laws for Gas Conversions
Gas laws, like the Ideal Gas Law (PV=nRT), are crucial for converting between gas volume and moles:
- Volume (V), Pressure (P), Temperature (T), and the number of moles (n) are interlinked.
- When temperature and pressure are constant, you can use the relationship V1/n1 = V2/n2.
Example:
If you have 4 liters of an ideal gas at STP (Standard Temperature and Pressure), how many moles of gas are present?
- Using the molar volume of a gas at STP (22.4 L/mol):
- 4 L * (1 mol / 22.4 L) ≈ 0.178 mol
Tip 5: Account for the Purity and Percentage Composition

When working with real-world samples, consider:
- The purity of the substance which affects the effective molar mass.
- The percentage composition of elements in a compound to determine moles.
For instance, if a sample of copper sulfate (CuSO4) has 95% purity, you would need to account for this when calculating moles:
- Given a 250 g sample of 95% pure CuSO4:
- Effective mass = 250 g * 0.95 = 237.5 g
- Molar mass of CuSO4 = 159.61 g/mol
- Moles of pure CuSO4 = 237.5 g / 159.61 g/mol ≈ 1.488 mol
These five tips are not just theoretical knowledge but practical tools that, once mastered, will make mole conversions almost second nature. By understanding and applying dimensional analysis, stoichiometry, molarity, gas laws, and accounting for substance purity, you'll solve problems with speed and accuracy.
What is the importance of Avogadro’s number in mole conversion?

+
Avogadro’s number (6.022 x 1023) allows us to link the macroscopic measurements, like the mass of a sample, to the microscopic scale of atoms and molecules. It’s essentially a conversion factor that helps in understanding the scale between moles and particles.
Why is stoichiometry essential in chemistry?

+
Stoichiometry provides the numerical relationship between reactants and products in a chemical reaction. It’s critical for predicting yields, understanding reaction pathways, and scaling up reactions for industrial purposes.
How does the purity of a substance affect mole calculations?
+
Impurities dilute the molar mass of the substance, so when calculating moles, you must use the effective mass of the pure component of the substance to avoid overestimating the number of moles present.