5 Essential Tips for Mole Calculations

Calculating moles is a fundamental skill in chemistry, pivotal for various reactions, stoichiometry, and understanding the composition of substances. Whether you're studying for an exam, working on a lab report, or just delving into the mysteries of chemistry, mastering mole calculations can significantly enhance your grasp of chemical principles. Here, we'll explore five essential tips to help you navigate through mole calculations with ease and precision.
Understand the Definition of a Mole

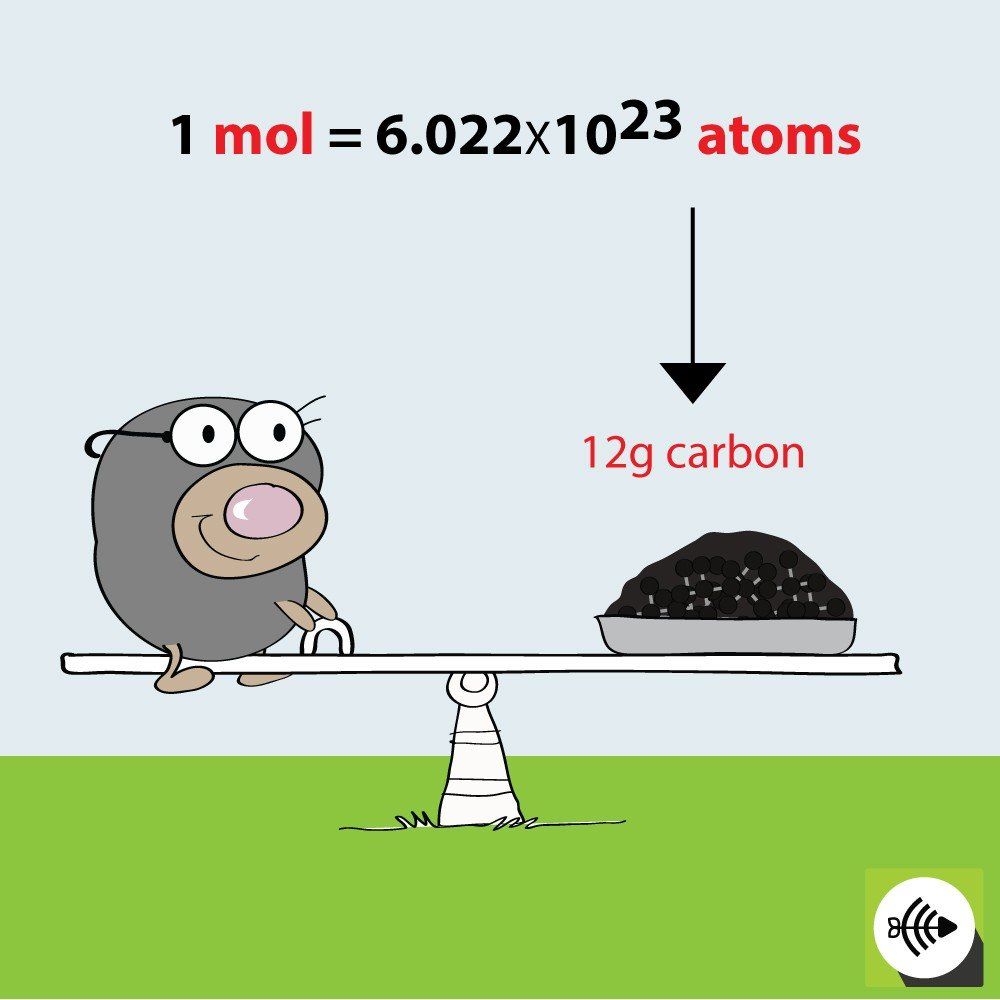
The mole is defined as Avogadro’s number, approximately 6.022 × 10^23, which represents the number of atoms in a mole of a substance. This relationship is crucial:
- Mass-Mole-Molecule: 1 mole of any element or compound contains the same number of atoms or molecules as one mole of hydrogen atoms.
- Molar Mass: The mass of one mole of a substance in grams is known as its molar mass, which can be found on the periodic table.

Use Molar Mass Correctly
To convert between grams and moles:
- Use the formula Mass in grams / Molar Mass in g/mol = Moles. For example, if you have 36 grams of carbon ©, its molar mass is approximately 12 g/mol:
⚠️ Note: Always double-check your periodic table for the most accurate molar mass.
| Element | Atomic Mass (u) | Molar Mass (g/mol) |
|---|---|---|
| Hydrogen | 1.00784 | 1.00784 |
| Carbon | 12.011 | 12.011 |
| Oxygen | 15.999 | 15.999 |

Apply Dimensional Analysis

Dimensional analysis is your best friend when converting between different units in chemistry:
- Convert from grams to moles by multiplying by the inverse of molar mass (1/molar mass).
- Convert from moles to grams by multiplying by the molar mass.
Here’s an example for converting 0.5 moles of water (H₂O) to grams:
- 1 mole of H₂O has a molar mass of 18.015 g/mol. Therefore, 0.5 moles of H₂O is:
- 0.5 moles × 18.015 g/mol = 9.0075 grams
Convert Between Moles and Molecules
To convert moles to the number of molecules or vice versa:
- Multiply by Avogadro’s number (6.022 × 10^23) to go from moles to molecules.
- Divide by Avogadro’s number to convert molecules to moles.
This conversion is straightforward:
- Moles × Avogadro’s number = Number of molecules
Use Stoichiometry

Stoichiometry is the mathematical relationship between the quantities of reactants and products in a chemical reaction. Here are some key points:
- Write a balanced equation to ensure the moles balance.
- Use the coefficients in the balanced equation to find the mole ratio between reactants and products.
For example, consider the reaction:
- N₂ + 3H₂ → 2NH₃
- If you start with 2 moles of N₂, you’ll need 6 moles of H₂ to produce 4 moles of NH₃.
In conclusion, mastering mole calculations opens up a world of chemical understanding, from accurately measuring substances for experiments to predicting the outcomes of chemical reactions. Remember these tips:
- Understand the definition and use of Avogadro's number.
- Correctly apply molar mass in your calculations.
- Employ dimensional analysis for unit conversion.
- Know how to convert between moles and the number of particles.
- Practice stoichiometry for reaction calculations.
By following these guidelines, you'll enhance your chemistry proficiency and gain confidence in handling mole-based problems.
Why do I need to understand moles?

+
Moles provide a universal way to count atoms or molecules in a substance, essential for predicting reaction outcomes and understanding chemical composition.
What’s the difference between molar mass and atomic mass?

+
Atomic mass is the mass of a single atom, typically expressed in atomic mass units (u). Molar mass, on the other hand, is the mass of one mole of a substance, usually in grams per mole (g/mol).
How do I convert grams to moles if I don’t know the molar mass?

+
You need the molar mass to perform this conversion. This information can be found on the periodic table or through calculation if the substance’s formula is known.